Consider the hypothetical reaction, A 2 + B 2 2AB, where the rate law is: The
Question:
Consider the hypothetical reaction, A2 + B2 → 2AB, where the rate law is:
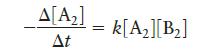
The value of the rate constant at 302°C is 2.45 × 10-4 L mol-1 s-1, and at 508°C the rate constant is 0.891 L mol-1 s-1. What is the activation energy for this reaction? What is the value of the rate constant for this reaction at 375°C?
Transcribed Image Text:
Δ[Α2] k[Ag][B2] ΔΕ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a The activation energy for t...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the hypothetical reaction A + B + 2C 2D + 3E In a study of this reaction, three experiments were run at the same temperature. The rate is defined as 2d[B]/dt. Experiment 1: [A]0 = 2.0 M [B]0...
-
Consider the hypothetical reaction B E + F which is assumed to occur by the mechanism Where B* represents a B molecule with enough energy to surmount the reaction energy bar70. Consider the following...
-
Consider the hypothetical reaction A + B + 2C 2D + 3E where the rate law is An experiment is carried out where [A]0 = 1.0 Ã 10-2 M, [B]0 = 3.0 M, and [C]0 = 2.0 M. The reaction is started, and...
-
Suppose that in a particular area the consumption of water varies tremendously throughout the year, with average household summer use exceeding winter use by a great deal. What effect would this have...
-
The diameters of fully grown white oak trees are normally distributed, with a mean of 3.5 feet and a standard deviation of 0.2 foot, as shown in the figure. Random samples of size 16 are drawn from...
-
Solve each of the following equations. 3 (3 2) - (4x-3) = 11 +3x 60
-
Do heavier people burn more energy? The study of dieting described in Exercise 10 collected data on the lean body mass (in kilograms) and metabolic rate (in calories) for 12 female and 7 male...
-
How does operating income differ from net income? How do operating assets differ from total assets? What is the advantage in removing non-operating items from the DuPont analysis?
-
ABC manufacturer of poster stands. Every stand passes through two departments: the assembly department and the finishing department. This problem focuses on the assembly department. The...
-
The auditors of Landi Corporation wish to use a structured approach to nonstatistical sampling to evaluate the reasonableness of the accounts receivable. Landi has 15,000 receivable accounts with a...
-
A certain substance, initially present at 0.0800 M, decomposes by zero-order kinetics with a rate constant of 2.50 102 -2 mol L -1 s - 1 . Calculate the time (in seconds) required for the system to...
-
The reaction A(aq) + B(aq) products(aq) was studied, and the following data were obtained: What is the order of the reaction with respect to A? What is the order of the reaction with respect to B?...
-
Modify the VHDL design in Figure 4-58 so that z = 1 only when the digital value is less than 1010 2 . Figure 4-58
-
Solve xy' - 2y = x.
-
Vanishing Games Corporation (VGC) operates a massively multiplayer online game, charging players a monthly subscription of $15. At the start of January 2021, VGC's income statement accounts had zero...
-
Financial data, medical service data, and staffing data are best defined as what type of data
-
. What is meant by the term "foreign exchange rate"? In general, what causes foreign exchange rates to vary over time? . Do changes in foreign exchange rates benefit or hurt U.S. companies that are...
-
Primare Corporation has provided the following data concerning last month's manufacturing operations. Purchases of raw materials Indirect materials used in production Direct labor Manufacturing...
-
Fill in the blank(s) to correctly complete each sentence. The range of the relation in Exercise 1 is _____________.
-
Factor and simplify, if possible. Check your result using a graphing calculator. 3 cot 2 + 6 cot + 3
-
A 1.75 mole sample of an ideal gas is compressed isothermally from 62.0 L to 19.0 L using a constant external pressure of 2.80 atm. Calculate q, w, U, and H.
-
For each of the compounds below, locate the lone pair adjacent to a positive charge and draw the resonance structure: a. b. c. N.
-
Assume the following simplified dependence of the pressure in a ventricle of the human heart as a function of the volume of blood pumped. P s , the systolic pressure, is 120. mm Hg, corresponding to...
-
Accounting changes fall into one of three categories. Identify and explain these categories and give an example of each one.
-
Machinery is purchased on May 15, 2015 for $120,000 with a $10,000 salvage value and a five year life. The half year convention is followed. What method of depreciation will give the highest amount...
-
Flint Corporation was organized on January 1, 2020. It is authorized to issue 14,000 shares of 8%, $100 par value preferred stock, and 514,000 shares of no-par common stock with a stated value of $2...

Study smarter with the SolutionInn App


