Consider the reaction Calculate the heat when (100.0 mathrm{~mL}) of (0.500 mathrm{M} mathrm{HCl}) is mixed with (300.0
Question:
Consider the reaction
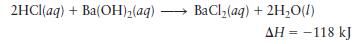
Calculate the heat when \(100.0 \mathrm{~mL}\) of \(0.500 \mathrm{M} \mathrm{HCl}\) is mixed with \(300.0 \mathrm{~mL}\) of \(0.100 \mathrm{M} \mathrm{Ba}(\mathrm{OH})_{2}\). Assuming that the temperature of both solutions was initially \(25.0^{\circ} \mathrm{C}\) and that the final mixture has a mass of \(400.0 \mathrm{~g}\) and \(\mathrm{a}\) specific heat capacity of \(4.18 \mathrm{~J}^{\circ} \mathrm{C}^{-1} \mathrm{~g}^{-1}\), calculate the final temperature of the mixture.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





