Determine whether titanium dioxide can be reduced by carbon at 1000. K in each of the following
Question:
Determine whether titanium dioxide can be reduced by carbon at 1000. K in each of the following reactions: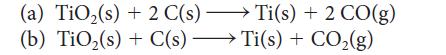
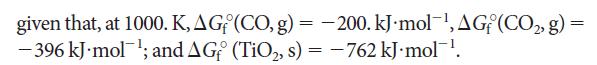
Transcribed Image Text:
(a) TiO₂(s) + 2 C(s) →→→ Ti(s) + Ti(s) + 2 CO(g) - (b) TiO₂(s) + C(s) Ti(s) + CO₂(g) -
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
To determine whether titanium dioxide TiO2 can be reduced by carbon C at 1000 K in each of the given ...View the full answer

Answered By

HILLARY KIYAYI
I am a multi-skilled, reliable & talented Market analysis & Research Writer with a proven ability to produce Scholarly Papers, Reports, Research and Article Writing and much more. My ultimate quality is my English writing/verbal skill. That skill has proven to be the most valuable asset for project writing, Academic & Research writing, Proofreading, HR Management Writing, business, sales, and a variety of other opportunities.
4.80+
24+ Reviews
60+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) (b) (c) (d) ...
-
BACKGROUND You are an information analyst working for NEE. The company president has asked you to prepare a Quantitative analysis of financial, sales, and operations data to help determine which...
-
Each of the following reactions will be encountered at some point in this text. Classify each one according to whether the organic substrate is oxidized or reduced in the process. (a) CH3C = PCH +...
-
List four components and four guidelines that the J. Crew mission statement fails to exhibit. Write a new and improved mission for J. Crew.
-
Consider a potential problem in the half-space defined by z ? 0, with Dirichlet boundary conditions on the plane z = 0 (and at infinity). (a) Write down the appropriate Green function G(x, x'). (b)...
-
The four-bar mechanism of Prob. 6/108 is repeated here. The coupler AB has a mass of 7 kg, and the masses of crank OA and the output arm BC may be neglected. Determine and plot the torque M which...
-
In valuing illiquid interests in private businesses, why do appraisers normally begin with appraisals at the marketability minority level of value?
-
Describe the broad range of talent management efforts that use software applications by going to www.learn.com. Then give some examples of firms that have successfully used these applications.
-
Apple Corporation reported the following amounts at the end of the first year of operations: Common stock $150,000 Sales revenue $750,000 Total assets $550,000 Dividends declared $ 46,000 Total...
-
Which is the thermodynamically more stable iron oxide in air, Fe 3 O 4 (s) or Fe 2 O 3 (s)? Justify your selection.
-
Calculate the standard reaction entropy, enthalpy, and Gibbs free energy for each of the following reactions from data in Appendix 2A: (a) The production of synthesis gas, a low-grade industrial...
-
Every day after school, Trent volunteers at his neighborhood community center. He helps the young children with their homework and organizes sports activities. Trent really believes in the work that...
-
1 . Journalize the following transactions: ( a ) Issued 1 , 0 0 0 shares of $ 1 0 par common stock at $ 5 9 for cash. ( b ) Issued 1 , 4 0 0 shares of $ 1 0 par common stock in exchange for equipment...
-
Using alpha .05, determine if moving to a larger enclosure decreased tiger anxiety levels. You should first calculate the difference (After - Before) Tiger Before Anthony 45 45 Banthony 56 After 38...
-
Cyclohexane (C 6 H 12 ) is produced by mixing Benzene and hydrogen. A process including a reactor, separator, and recycle stream is used to produce Cyclohexane. The fresh feed contains 260L/min C 6 H...
-
Suppose the city is undergoing severe ination. Specifically, both goods prices have risen by 10%. What percentage of a raise in the wage rate should Alex request from her boss, for her to maintain...
-
1. An iron cube of mass 0.55 kg is raised to a temperature of 100C by being placed in boiling water for 5 minutes. It is then removed and transferred immediately to an aluminium calorimeter filled...
-
To detect bombs that may be smuggled onto airplanes, the Federal Aviation Administration (FAA) will soon require all major airports in the United States to install thermal neutron analyzers. The...
-
Extend Algorithms 3.4 and 3.5 to include as output the first and second derivatives of the spline at the nodes.
-
The structure of tartaric acid is a. Is the form of tartaric acid pictured below optically active? Explain. b. Draw the optically active forms of tartaric acid. HO,CCHCHCO,H
-
The structure of tartaric acid is a. Is the form of tartaric acid pictured below optically active? Explain. b. Draw the optically active forms of tartaric acid. HO,CCHCHCO,H
-
The structure of tartaric acid is a. Is the form of tartaric acid pictured below optically active? Explain. b. Draw the optically active forms of tartaric acid. HO,CCHCHCO,H
-
Minden Company introduced a new product last year for which it is trying to find an optimal selling price. Marketing studies suggest that the company can increase sales by 5,000 units for each $2...
-
Prepare the adjusting journal entries and Post the adjusting journal entries to the T-accounts and adjust the trial balance. Dresser paid the interest due on the Bonds Payable on January 1. Dresser...
-
Venneman Company produces a product that requires 7 standard pounds per unit. The standard price is $11.50 per pound. If 3,900 units required 28,400 pounds, which were purchased at $10.92 per pound,...

Study smarter with the SolutionInn App


