Determine which of the following compounds are stable with respect to decomposition into their elements under standard
Question:
Determine which of the following compounds are stable with respect to decomposition into their elements under standard conditions at 25°C (see Appendix 2A):
(a) PCl5(g);
(b) HCN(g);
(c) NO(g);
(d) SO2(g).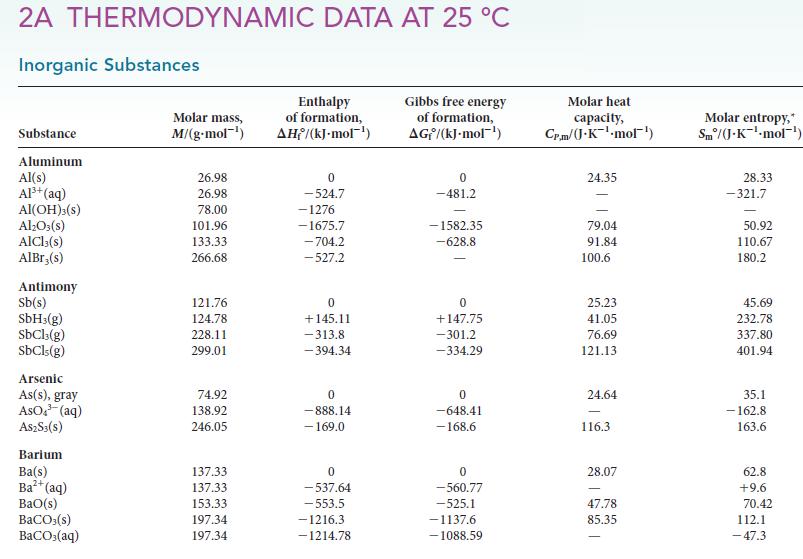
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) AI(OH)3(s) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO³(aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a and d ...View the full answer

Answered By

Ashwani Prasad
I had pursued B.E. in Chemical Engineering in 2018. I have worked as a Junior Engineer in the chemical industry. So, I know theoretical as well as the practical aspect of an engineering problem. I am keenly interested in Chemical process calculations, chemical reaction engineering and chemical technology.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Determine which of the following compounds are stable with respect to decomposition into their elements under standard conditions at 25C (see Appendix 2A): (a) C 3 H 6 (g), cyclopropane; (b) CaO(s);...
-
The term thermodynamic stability refers to the sign of r G . If r G is negative, the compound is stable with respect to decomposition into its elements. Use the data in Appendix D to determine...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Kaspar Corporation makes a commercial-grade cooking griddle. The following information is available for Kaspar Corporation's anticipated annual volume of 25,600 units. Per Unit Total Direct materials...
-
A point charge q is brought to a position a distance d away from an infinite plane conductor held at zero potential. Using the method of images, find: (a) The surface-charge density induced on the...
-
The motion of the outer frame B is given by x B = b sin wt. For what range of the driving frequency w is the amplitude of the motion of the mass m relative to the frame less than 2b? XB = b sin ot...
-
What are the sources of value at the shareholder (i.e., nonmarketable minority interest) level of value?
-
A planned factory expansion project has an estimated initial cost of $800,000. Based on a discount rate of 20 percent, the present value of the future cost savings from the expansion is $843,000....
-
Weighted Average Method, Unit Cost, Ving Invents Ce Inc., manufactures products that pass through two or more processen. During lune, equivalent units were computed using the weighted avec method...
-
Draw the Lewis structure for the hypothetical molecule N 6 , consisting of a six-membered ring of nitrogen atoms. Using bond enthalpies, calculate the enthalpy of reaction for the decomposition of N...
-
The following picture shows a molecular visualization of a system undergoing a spontaneous change. Account for the spontaneity of the process in terms of the entropy changes in the system and the...
-
Lamp Light Limited (LLL) in E9-11 calculates a fixed overhead rate based on budgeted fixed overhead of $32,400 and budgeted production of 24,000 units. Actual results were as follows: Number of units...
-
Ted sold his Microsoft stock for $40,000 paying a commission of $800. He purchased the stock in 2004 for $8,000 and paid commission of $200. What is the recognized gain on the sale?
-
Liquid water at 80C and at 1atm flows through a heated pipe at a flow rate of 3.1 kg/s. It then leaves the pipe as steam. The water receives 9753840 J of heating from the pipe. Calculate the...
-
The balance sheet of River Electronics Corporation as of December 31, 2023, included 14.00% bonds having a face amount of $90.7 million. The bonds had been issued in 2016 and had a remaining discount...
-
The term mutually exclusive means that two events have no common elements in them. The occurrence of one event means that the other other event does not occur. An example of a mutually exclusive...
-
9a A conical pendulum is made by hanging a mass of 5.0 kg from a large spring of length 1.0 m and spring constant k = 100 N/m. The spring moves in a circle at an angle of 25 deg. When at rest hanging...
-
Write complete nuclear equations for the following processes: (a) Tritium, 3H, undergoes decay; (b) 242Pu undergoes -particle emission; (c) 131I undergoes decay; (d) 251Cf emits an particle.
-
The registrar of a college with a population of N = 4,000 full-time students is asked by the president to conduct a survey to measure satisfaction with the quality of life on campus. The following...
-
Reagents such as HCl, HBr, and HOH (H 2 O) can add across carboncarbon double and triple bonds, with H forming a bond to one of the carbon atoms in the multiple bond and Cl, Br, or OH forming a bond...
-
Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C 4 H 8 . Another common feature of alkenes and cycloalkanes is that both have restricted rotation about...
-
Alkenes and cycloalkanes are structural isomers of each other. Give an example of each using C 4 H 8 . Another common feature of alkenes and cycloalkanes is that both have restricted rotation about...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


