Does the information on alkali metals in Table 12.9 of the text confirm the general periodic trends
Question:
Does the information on alkali metals in Table 12.9 of the text confirm the general periodic trends in ionization energy and atomic radius? Explain.
Table 12.9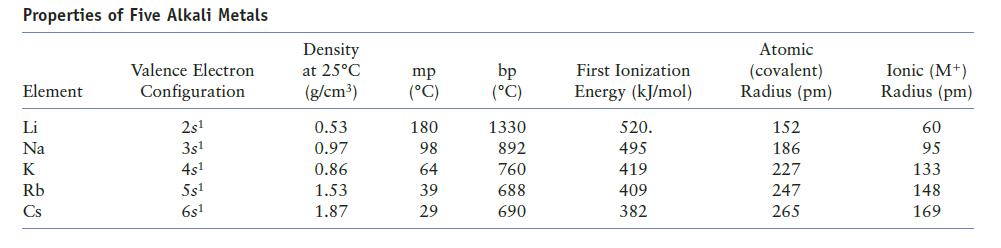
Transcribed Image Text:
Properties of Five Alkali Metals Element Li Na K Rb Cs Valence Electron Configuration 2s¹ 3s¹ 4s¹ 5s¹ 6s1 Density at 25°C (g/cm³) 0.53 0.97 0.86 1.53 1.87 mp (°C) 180 98 64 39 29 bp (°C) 1330 892 760 688 690 First Ionization Energy (kJ/mol) 520. 495 419 409 382 Atomic (covalent) Radius (pm) 152 186 227 247 265 Ionic (M+) Radius (pm) 60 95 133 148 169
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Answer The information on alkali metals in Table 129 does confirm the general ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The radius trend and the ionization energy trend are exact opposites. Does this make sense? Define electron affinity. Electron affinity values are both exothermic (negative) and endothermic...
-
Explain why the first ionization energy tends to increase as one proceeds from left to right across a period. Why is the first ionization energy of aluminum lower than that of magnesium and the first...
-
The ionization energy of O2 is smaller than the ionization energy of atomic O; the opposite is true for the ionization energies of N2 and atomic N. Explain this behavior in terms of the molecular...
-
a. Consider the general effect of the discount rate on the dynamic efficient allocation of a depletable resource across time. Suppose we have two versions of the two-period model discussed in this...
-
A veterinarian records the weights of dogs treated at a clinic. The weights are normally distributed, with a mean of 52 pounds and a standard deviation of 15 pounds. Find the weights x corresponding...
-
To earn some money, John is thinking of starting up a Back-Yard BBQ stand at his university campus this summer. The basic BBQ equipment will cost $2690 and the variable cost (VC) for each BBQ Meal is...
-
The range is completely determined by the two extreme scores in a distribution. The standard deviation, on the other hand, uses every score. a. Compute the range (choose either definition), the...
-
Able Control Company, which manufactures electrical switches, uses a standard cost system and carries all inventory at standard cost. The standard factory overhead cost per switch is based on direct...
-
Compute the principal for the loan. Use ordinary interest. (Round answer to the nearest cent) Principal Rate Time Interest 12.6% 160 days $896.00 Dodo - Format BIU A
-
Skop Inc. is a construction company specializing in custom patios. The patios are constructed of concrete, brick, fiberglass, and lumber, depending upon customer preference. On June 1, 2012, the...
-
Spectroscopists use emission spectra to confirm the presence of an element in materials of unknown composition. How is this possible?
-
The electron affinity for sulfur is more exothermic than that for oxygen. How do your account for this?
-
David Logan: Tribal Leadership16:36 minutes https://www.ted.com/talks/david_logan_on_tribal_leadership This video focuses on five kinds of tribes that people naturally form and how they influence...
-
List the model assumptions for one-way ANOVA and briefly explain how to assess them.
-
Remember that a correctly labeled graph requires that you label all axes, curves, and equilibrium point values. The word "calculate" means you must show your work. Assume the market for Good Z is in...
-
Answer the following questions by writing a paragraph or two in English. (a) [easy] Previously we defined probability as P(A) = Describe a situtation where this fails to produce the correct...
-
Find f''(x). f(x)=5x-14x- 612x f'(x)=
-
Simplify. 32-6 3-6
-
Solve each inequality. Give the solution set using interval notation. x 2 - 3x 5
-
Use translations to graph f. f(x) = x-/2 +1
-
You are told that 2.55 g of a gaseous hydrocarbon occupies a vessel of volume 3.00 L at 0.950 atm and 82.0C. Draw the Lewis structure of this hydrocarbon.
-
A chemist prepares a sample of hydrogen bromide and finds that it occupies 500. mL at 45C and 120. Torr. What volume would it occupy at 0 C at the same pressure?
-
The expression for the capillary rise in Exercise 3G.11 assumes that the tube is vertical. How will the expression be modified when the tube is held at an angle (theta) to the vertical? Exercise...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


