Draw the Lewis structure of boric acid, B(OH) 3 . (a) Is resonance important for its description?
Question:
Draw the Lewis structure of boric acid, B(OH)3.
(a) Is resonance important for its description?
(b) The proton transfer equilibrium for boric acid is given in a footnote to Table 6C.1. In that reaction does boric acid act as a Lewis acid, a Lewis base, or neither?
Justify your answer by using Lewis structures of boric acid and its conjugate base.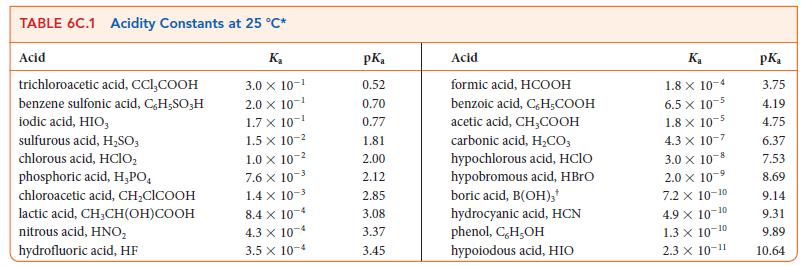
Transcribed Image Text:
TABLE 6C.1 Acidity Constants at 25 °C* Acid trichloroacetic acid, CCI,COOH benzene sulfonic acid, C,H-SO;H iodic acid, HIO, sulfurous acid, H₂SO3 chlorous acid, HClO₂ phosphoric acid, H,PO chloroacetic acid, CH₂ClCOOH lactic acid, CH,CH(OH)COOH nitrous acid, HNO₂ hydrofluoric acid, HF K₂ 3.0 X 10-¹ 2.0 × 10-¹ 1.7 X 10-¹ 1.5 X 10-² 1.0 x 10-² 7.6 X 10-³ 1.4 x 10-³ 8.4 x 10-4 4.3 x 10-4 3.5 x 10-4 pK₂ 0.52 0.70 0.77 1.81 2.00 2.12 2.85 3.08 3.37 3.45 Acid formic acid, HCOOH benzoic acid, C, H,COOH acetic acid, CH₂COOH carbonic acid, H₂CO3 hypochlorous acid, HClO hypobromous acid, HBrO boric acid, B(OH)3* hydrocyanic acid, HCN phenol, C,H,OH hypoiodous acid, HIO Ka 1.8 x 10-4 6.5 x 10-5 1.8 x 10-5 4.3 X 10-7 3.0 × 10-8 2.0 x 10-5 -9 7.2 x 10-10 4.9 X 10-¹ -10 1.3 × 10-10 2.3 × 10-11 pK₂ 3.75 4.19 4.75 6.37 7.53 8.69 9.14 9.31 9.89 10.64
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The Lewis structure of boric acid is H HOBOH HO Boric ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
In the vapor phase, BeCl2 exists as a discrete molecule. (a) Draw the Lewis structure of this molecule, using only single bonds. Does this Lewis structure satisfy the octet rule? (b) What other...
-
The following items are related to income tax expenses. Determine the specific eight or nine-digit codification citation (XXX-XX-XX-XX) for each of the following items. Please enter only the ASC...
-
Hydrogen peroxide, H 2 O 2 , is a nontoxic bleaching agent being used as a replacement for chlorine in industry and home laundries. The bleaching process is an oxidation, and when hydrogen peroxide...
-
trade on the common stock of Taz, Inc. that have a strike price of $ 51.00 and a premium of $ 1.00 . In each of the next four parts, calculate the net profit (or loss) on the option position. Note:...
-
Strategy balanced scorecard. Meredith Corporation makes a special-purpose machine, D4H, used in the textile industry. Meredith has designed the D4H machine for 2009 to be distinct from its...
-
What are five main functions of a database administrator?
-
Calculate the mean, median, and mode stock price.
-
Matulis, Inc., a C corporation, owns a single asset with a basis of $325,000 and a fair market value of $800,000. Matulis holds a positive E & P balance. It elects S corporation status and then sells...
-
The order of priority of claims in liquidation is firmly established in legal precedent. As depicted in Table 18.9 of the textbook, common shareholders are last in priority. Yet, in bankruptcy...
-
Outline a long-term approach that you would recommend for narrowing a credibility gap that might exist between an organizations Human Resources department and its department managers.
-
Draw the Lewis structure or symbol for each of the following species and identify each one as a Lewis acid or Lewis base: (a) NH3; (b) BF3; (c) Ag; (d) F; (e) H.
-
The pH of 0.40 m HF(aq) is 1.93. Calculate the change in pH when 0.356 g of sodium fluoride is added to 50.0 mL of the solution. Ignore any change in volume.
-
An astronomer discovers a new red star that emits light with maximum intensity at 632 nm. What is the temperature at the surface of the star?
-
On a dreary morning in May 1995, Paul found himself sitting on the floor of the hallway, crouched against the cold wall, feeling dejected and desperate. The bustling cacophony of the people in nearby...
-
Two speakers S1 and S2 are at a distance from each other. Point Q is located at y = 2.1 m above loudspeaker S2 while point P is located at x = 4.1 m in front of loudspeaker S1. The two loudspeakers...
-
Q1 Go to the Office of the Superintendent of Financial Institutions (OSFI) and find data (as of December 31, 2020) for all domestic banks on total liabilities, total deposits, and residual of assets...
-
Explain your viewpoint/philosophy on the Christian's responsibility to demonstrate wise stewardship of higher education resources. How should a believer handle the institution's finances? As you...
-
TranscribedText: 4. DQ 5. Create your initial post on the DQ 5 Discussion Board in response to the following: e There are many different opinions about how media, specifically TV and the Internet,...
-
A zoologist measured tail length in 86 individuals, all in the 1-year age group, of the deermouse Peromyscus. The mean length was 60.43 mm and the standard deviation was 3.06 mm. A 95% confidence...
-
When the concentration of a strong acid is not substantially higher than 1.0 10-7 M, the ionization of water must be taken into account in the calculation of the solution's pH. (a) Derive an...
-
Propose a plausible mechanism for each of the following transformations: (a) (b) I-CI AICI3 CH,Cl, AICI,
-
Propose a plausible mechanism for the following transformation: NaOMe + Nacl OMe CI
-
Predict the product(s) of the following reactions: (a) (b) (c) (d) 1) HNO3, H,SO, 2) Zn, HCI Br
-
(International Finance) Computing a Currency changes = (e1 - e0 )/ e0 where e0 = old currency value e1 = new currency value (a) If the dinar devalues against the U.S. dollar by 45%, the U.S. dollar...
-
2. Fill in the time line for the Sawing Department. Use the time line to help you compute the number of equivalent units and the cost per equivalent unit in the Sawing Department for September Show...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...

Study smarter with the SolutionInn App


