Estimate the vapor pressure of heavy water, D 2 O, and of normal water at 25C by
Question:
Estimate the vapor pressure of heavy water, D2O, and of normal water at 25°C by using data in Appendix 2A. How do these values compare with each other? Using your knowledge of intermolecular forces and any quantum effects, such as the zero point energy of intermolecular vibrations, explain the reason for the difference observed.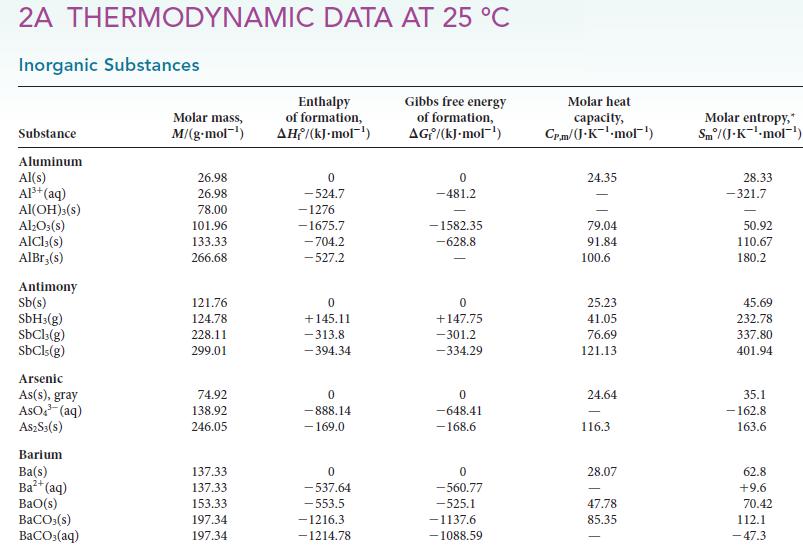
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO³(aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g-mol ¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K-¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
For HO PH0 0...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Appendix B lists the vapor pressure of water at various external pressures. (a) Plot the data in Appendix B, vapor pressure (torr) versus temperature (oC). From your plot, estimate the vapor pressure...
-
In Section 11.5 we defined the vapor pressure of a liquid in terms of an equilibrium. (a) Write the equation representing the equilibrium between liquid water and water vapor and the corresponding...
-
Calculate the vapor pressure of water at 150C using the van der Waals equation of state. Repeat the calculation at 370C. How do these values compare with values obtained with the IF97 model?
-
The table shows equivalent temperatures in degrees Celsius and degrees Fahrenheit. (a) Plot the data with Fahrenheit temperature on the x-axis and Celsius temperature on the y-axis. What type of...
-
The diffusivity of toluene in air was determined experimentally by allowing liquid toluene to vaporize isothermally into air from a partially filled vertical tube 3 mm in diameter. At a temperature...
-
Markus Company's common stock sold for $4.25 per share at the end of this year. The company paid a common stock dividend of $0.68 per share this year. It also provided the following data excerpts...
-
Prepare a manufacturing statement and explain its purpose and links to financial statements
-
At the market close on October 27 of a recent year, McDonalds Corporation had a closing stock price of $93.49. In addition, McDonalds Corporation had a dividend per share of $2.44 during the previous...
-
Julie earned $165,000 working as a CPA last year. The total contributions to her DCPP last year were $16,200. Her DCPP contributions earned income of $600 during the year. Julie also maintains an...
-
Crane Medical manufactures hospital beds and other institutional furniture. The company's comparative balance sheet and income statement for 2019 and 2020 follow. Assets Current assets Crane Medical...
-
A reaction vessel is filled with Cl 2 (g) at 1.00 bar and Br 2 (g) at 1.00 bar, which are allowed to react at 1000. K to form BrCl(g)according to the equation Br 2 (g) + Cl 2 (g) 2 BrCl(g), K = 0.2....
-
The gas phosphine, PH 3 , decomposes by the reaction 2 PH 3 (g) 2 P(s) + 3 H 2 (g). In an experiment, pure phosphine was placed in a rigid, sealed flask of volume 1.00 L at 0.64 bar and 298 K. After...
-
a. What is the cost per camera (ignoring taxes) for Edwards Electronics and for Sears? b. For each store, what is the minimum selling price required to cover cost, over-head, and desired profits? c....
-
1. Do you think that the NFL and franchise owners are meeting their obligations to employee health and safety? 2. Do you think that the NFL's and owners' responsibilities in terms of player safety...
-
Explain the term \'management\'. Also, explain briefly mission functions of management. ( b ) What are the different types of plant layout? Explain any two with neat sketches.
-
Suppose that you are considering an investment product that promises to pay $ 2 , 0 0 0 at the end of each year for the next five years. Assume that a discount rate of 1 2 % is applicable to similar...
-
Leadership Philosophy: Democratic and Transformational leadership In 700+ words ,explain how the leadership philosophy might impact an organization and how it would be beneficial.Identify what are...
-
performance and participation. The employee requirement that is met is status and recognition. The performance result is awakened drives. This model is dependent on leadership strive. It gives a...
-
A water main made of rough concrete loses 43 psi (lb/in.2) of pressure over a 1-mi horizontal length. Estimate the size of the pipe required to carry 16.5 cfs (ft3 /sec) of water at 68F (20oC Assume...
-
Write the expression in radical notation. Then evaluate the expression when the result is an integer. 23 -1/2
-
Propose the structure of a compound that exhibits the following 1 H NMR data: (a) C 5 H 10 O 1.09 (6H, doublet) 2.12 (3H, singlet) 2.58 (1H, septet) (b) C 5 H 12 O 0.91 (3H, triplet) 1.19 (6H,...
-
A compound with molecular formula C 8 H 10 O produces six signals in its 13 C NMR spectrum and exhibits the following 1 H NMR spectrum. Deduce the structure of the compound. Proton NMR Chemical Shift...
-
Deduce the structure of a compound with molecular formula C 9 H 12 that produces the following 1 H NMR spectrum: Proton NMR Chemical Shift (ppm)
-
Aecerty 1067687 was completed with the folowing charaderistick Murulectere sec00 5xs:99 s35ida sputed
-
Assume todays settlement price on a CME EUR futures contract is $1.3180 per euro. You have a long position in one contract. EUR125,000 is the contract size of one EUR contract. Your performance bond...
-
Q2. Company ABC bought an equipment for $20,000 in 2015, with useful life of 5 years $5,000 residual value amortized using straight-line method. Prepare a table to illustrate the differences...

Study smarter with the SolutionInn App


