Ethanol is a renewable and clean-burning component of gasoline: What is the change in internal energy for
Question:
Ethanol is a renewable and clean-burning component of gasoline: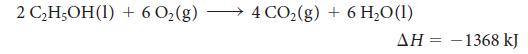
What is the change in internal energy for the reaction of 1.00 mol C2H5OH(l)?
Transcribed Image Text:
2 CHOH(I) + 602(g) - 4 CO2(g) + 6 H,O(1) ΔΗ -1368 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
The change in internal energy AH for the given reaction is 1368 kJmol The relationship between the c...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
A sample consisting of 22.7 g of a nongaseous, unstable compound X is placed inside a metal cylinder with a radius of 8.00 cm, and a piston is carefully placed on the surface of the compound so that,...
-
The standard enthalpy of formation of H2O(l) at 298 K is 285.8 kJ/ mol. Calculate the change in internal energy for the following process at 298 K and 1 atm: H2O(l) H2(g) + O2(g) Eo = ?
-
Violet Flowers expects to sell 3,000 plants a month. She estimated the following monthly costs: Variable Costs $9,000 Fixed Costs $15,000 During her second month of operation, Violet would like to...
-
SEK Printing provides printing services to many different corporate clients. Although SEK bids most jobs, some jobs, particularly new ones, are negotiated on a cost-plus basis. Cost-plus means that...
-
Evaluate the integral. SVi - sin x dx
-
2. Calculate the balances that should be in the three capital accounts on January 1, 2019, taking into account the corrections that must be made for errors made in the calculation of income in the...
-
The account balances of Wilford Towing Service at June 30, 2016, follow Equipment...........$ 17,500 Office Supplies ........... 1,300 Notes Payable........... 6,900 Rent Expense........... 800...
-
Question Jackie has been offered positions by two cable companies. The first company pays a salary of $ 1 4 , 0 0 0 plus a commission of $ 1 0 0 for each cable package sold. The second pays a salary...
-
Assuming that the heat capacity of an ideal gas is independent of temperature, calculate the entropy change associated with lowering the temperature of 4.10 mol of ideal gas atoms from 225.71C to...
-
The bond enthalpy in NO is 632 kJ mol 1 and that of each NO bond in NO 2 is 469 kJ mol 1 . Using Lewis structures and the data in Table 4E.3, explain (a) The difference in bond enthalpies between...
-
A compound epicyclic gear train is shown in Fig.15.48 The gears \(A, D\) and \(E\) are free to rotate on axis \(P\). The compound gear \(B\) and \(C\) rotate together on axis \(Q\) at the end of arm...
-
Time ( s ) Velocity ( m / s ) 1 2 3 4 5 6 7 8 Calculate the velocity
-
The table below gives the data about Etruria's balance of payments. (All figures are in billions of dollars.) Foreign investment in Etruria Secondary (transfers) income received from abroad Primary...
-
Olive Corporation buys a material for P20 per unit. Sixteen thousand parts a year are needed. Carrying costs is P3.00 per unit and the ordering cost is P15. Required: Compute the economic order...
-
As a healthcare leader or manager, most of us are charged with supervising employees. The literature suggests the importance of hiring and retaining employees with high levels of emotional...
-
7-8. Evaluate the sum exactly. (10 points each) 7. 18 (1) n (33) "
-
A true-breeding rooster with a rose comb, feathered shanks, and cock-feathering was crossed to a hen that is true-breeding for pea comb and unfeathered shanks but is heterozygous for hen-feathering....
-
Write a program to move a signed number from smaller register to bigger register. Hint: movzx ax, bl Topic: Data Related Operators and Directives in assembly language
-
A sample of S8(g) is placed in an otherwise empty, rigid container at 1325 K at an initial pressure of 1.00 atm, where it decomposes to S2(g) by the reaction S8(g) 4S2(g) At equilibrium, the partial...
-
At a particular temperature, 12.0 moles of SO3 is placed into a 3.0- L rigid container, and the SO3 dissociates by the reaction 2SO3(g) 2SO2(g) + O2(g) At equilibrium, 3.0 moles of SO2 is present....
-
At 900oC, Kp = 1.04 for the reaction CaCO3(s) CaO(s) + CO2(g) At a low temperature dry ice (solid CO2), calcium oxide, and calcium carbonate are introduced into a 50.0- L reaction chamber. The...
-
Assignment Title: The Role of Bookkeeping in Business Management and Financial Reporting Objective: Understand the importance of proper bookkeeping procedures in the management of...
-
17) The adjustment that is made to allocate the cost of a building over its expected life is called:A) depreciation expense.B) residual value.C) accumulated depreciation.D) None of the above answers...
-
9) Prepaid Rent is considered to be a(n):A) liability.B) asset.C) contra-asset.D) expense.10) As Prepaid Rent is used, it becomes a(n):A) liability.B) expense. C) contra-asset.D) contra-revenue.11)...

Study smarter with the SolutionInn App


