Fill in the missing information in the following table. Solution a Solution b Solution c Solution d
Question:
Fill in the missing information in the following table.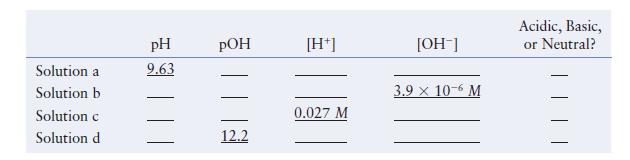
Transcribed Image Text:
Solution a Solution b Solution c Solution d pH 9.63 POH 12.2 [H+] 0.027 M [OH-] 3.9 x 10-6 M Acidic, Basic, or Neutral?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Solution A PH 963 pOH 14 963 437 H 10963 239 x 1010 M OH 10437 714 x 105 M Acidic Basic or Neu...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Fill in the missing information in the following table for a non-dividend-paying stock and European calloptions. Call A | 100 | 98 | 2% | 0.03 B | 100 | 98 | 2% | 0.04 C | 100 | 98 | 3% | 0.03 D |...
-
Fill in the missing information in the following table representing performance on an exam that is normally distributed with XÌ = 72 and S = 9. z-Score Percentile Rank 73 Ken 1.55 Drew Cecil 82
-
Fill in the missing information in the following table. Assume that Portfolio AB is 30 percent invested in StockA. Stock A 11% 37 21 26 13 Stock B Portfolio AB Year 2003 2004 2005 2006 2007 Average...
-
Suppose the following game is repeated once (that is, played twice in total). Bridget SB (10, 74) SA Alex S'A SA SB (70,70) (74, 10) (-10,-10) (40,40) (-10,-10) SB (-10,-10) (-10,-10) (50, 50) (a)...
-
Suppose that the weather can be only sunny or cloudy and the weather conditions on successive mornings form a Markov chain with stationary transition probabilities. Suppose also that the transition...
-
Halles Berry Farm establishes a $200 petty cash fund on September 4 to pay for minor cash expenditures. The fund is replenished at the end of each month. At the end of September, the fund contains...
-
47. LaMont works for a company in downtown Chicago. The company encourages employees to use public transportation (to save the environment) by providing them with transit passes at a cost of $270 per...
-
The following information was extracted from the December 31, 2011, current asset section of the balance sheets of four different companies: There were no transaction in short-term equity securities...
-
9. Rent scenarios a. Linda Peddler owns a bicycle factory. She could either rent or buy a machine to increase her factory's production capacity. She can rent the machine for its expected life of 10...
-
In a combustion chamber, oxygen diffuses through air to the carbon surface where it reacts to make CO and/or CO 2 . The mole fraction of oxygen at x=0 is 0.21. The reaction at the surface may be...
-
Can the pH of a solution be negative? Explain. True or false: A strong acid solution always has a lower pH than a weak acid solution. Explain.
-
You are asked for the H + concentration in a solution of NaOH(aq). Because sodium hydroxide is a strong base, can we say there is no H + , since having H + would imply that the solution is acidic?
-
Chloe Parker has accumulated substantial wealth and plans to gift some of her wealth to her son, Jack. She is considering two assets: a beach house, which cost $300,000 twenty years ago and now has a...
-
Dr. Powers operates a single-provider family medical practice. One medical assistant handles appointments, basic bookkeeping functions, and assists with medical records. Two additional medical...
-
Quiz 6 Fall 2019 - MGCR-211-001/002/003 edugen.wileyplus.com WileyPLUS Financial Accounting, Seventh Canadian Edition by Kimmel, Weygandt, Kieso, Trenholm, Irvine, and Burnley Help | System...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
4 Listen Using the DCF approach yields the value of the company as a whole. How would one refine this to determine the value of a share of stock? 1) Divide the company value by total assets. 2)...
-
The "is" or "is not" test established in McPhail v. Doulton (1971) for discretionary trusts creates more problems than it resolves.' Critically evaluate this statement. requirement Table of content...
-
Why are the days on Mercury very hot and the nights very cold?
-
How does health insurance risk differ from other types of insurance risk (e.g., automobile or homeowners insurance)? What is the difference between cost sharing and cost shifting? Is retiree health...
-
A flask that can withstand an internal pressure of 2500 torr, but no more, is filled with a gas at 21.0 o C and 758 torr and heated. At what temperature will it burst?
-
A gas sample containing 1.50 moles at 25 o C exerts a pressure of 400. torr. Some gas is added to the same container, and the temperature is increased to 50 o C. If the pressure increases to 800....
-
Consider the following chemical equation: 2NO 2 (g) N 2 O 4 (g) If 25.0 mL of NO 2 gas is completely converted to N 2 O 4 gas under the same conditions, what volume will the N 2 O 4 occupy?
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


