For enzyme-catalyzed reactions that follow the mechanism a graph of the rate as a function of [S],
Question:
For enzyme-catalyzed reactions that follow the mechanism
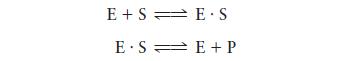
a graph of the rate as a function of [S], the concentration of the substrate, has the following general appearance:
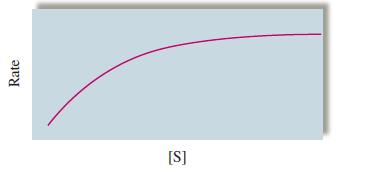
Note that at high substrate concentrations the rate no longer changes with \([S]\). Suggest a reason for this.
Transcribed Image Text:
E+SE.S E SE + P
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The graph you are referring to is a classic representation of enzyme kinetics known as the MichaelisMenten curve This curve shows the relationship bet...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
Refer to the facts presented in problem P7-13. In problem On January 25, 2011, Douglas Ltd. purchased 1,000 common shares of BMO (Bank of Montreal) for $65 each. During the remainder of 2011, Douglas...
-
The figure shows a circuit containing an electromotive force, a capacitor with a capacitance of C farads (F), and a resistor with a resistance of R ohms ( ). The voltage drop across the capacitor is...
-
Until recent years, labour cost constitutes a major portion of the total production cost. True/False
-
Sweetness of orange juice. Refer to the study of the quality of orange juice produced at a juice manufacturing plant, Exercise 11.32 (p. 629). Recall that simple linear regression was used to predict...
-
Refer to the Fit World situation in Problem 6-31A. Requirement 1. Using the results from the LIFO costing method calculations in Problem 6-31A, prepare a multi-step income statement for Fit World for...
-
thanks in advance, and happy holidays! Problem 24 The Hovious Inn is struggling to understand its unfavorable budget variance for cleaning rooms. The data are as follows: Time to Clean Hourly Wage...
-
Hydrogen peroxide decomposes to water and oxygen gas with the aid of a catalyst \(\left(\mathrm{MnO}_{2}ight)\). The activation energy of the uncatalyzed reaction is \(70.0 \mathrm{~kJ} /...
-
Reactions that require a metal catalyst are often zero order after a certain amount of reactants are present. Explain.
-
Ammonia at 0C, quality 60% is contained in a rigid 200-L tank. The tank and ammonia is now heated to a final pressure of 1 MPa. Determine the heat transfer for the process.
-
Finding Confidence Intervals. In Exercises 9-16, assume that each sample is a simple random sample obtained from a population with a normal distribution. Professor Evaluation Scores Listed below are...
-
Pacifico Company, a U . S . - based importer of beer and wine, purchased 1 , 7 0 0 cases of Oktoberfest - style beer from a German supplier for 4 5 9 , 0 0 0 euros. Relevant U . S . dollar exchange...
-
7.C. a. When you add two vectors you get another vector: yes or no? b. When you subtract two vectors you get another vector: yes or no? c. Given the coordinate system below where increasing numbers...
-
Problem 1 At a given instant, the position of a plane at A and a train at B are measured relative to a radar antenna at O. Determine the distance d between A and B at this instant. To solve the...
-
The Bell-Boeing V-22 Osprey tiltrotor is both an airplane and a helicopter. It's advantage is the ability to rotate its engines and rotors to vertical position for take-off, landings, and helicopter...
-
Suppose that individuals of type b reduce the per capita growth rate of type a by half as much as individuals of type a, and that individuals of type a reduce the per capita type b growth rate by...
-
State whether each of the following will increase or decrease the power of a one-way between-subjects ANOVA. (a) The effect size increases. (b) Mean square error decreases. (c) Mean square between...
-
For each of the following molecules or ions, draw the Lewis structure, list the number of lone pairs on the central atom, identify the shape, and estimate the bond angles: (a) PBr 5 ; (b) XeOF 2 ;...
-
The hybrid orbital h 1 = s + p x + p y + p z referred to in Exercise 2F.19 is not normalized. Find the normalization factor N , given that all the atomic orbitals are normalized to 1. A wavefunction ...
-
Using only Lewis structures that obey the octet rule, draw the Lewis structures and determine the formal charge on each atom in (a) SO 2 ; (b) SO 3 ; (c) SO 3 2 .
-
Famas Llamas has a weighted average cost of capital of 8.8 percent. The companys cost of equity is 12 percent, and its pretax cost of debt is 6.8 percent. The tax rate is 22 percent. What is the...
-
The common stock of a company paid 1.32 in dividens last year. Dividens are expected to gros at an 8 percent annual rate for an indefinite number of years. A) If the company's current market price is...
-
(1 point) Bill makes annual deposits of $1900 to an an IRA earning 5% compounded annually for 14 years. At the end of the 14 years Bil retires. a) What was the value of his IRA at the end of 14...

Study smarter with the SolutionInn App


