Given the following data: P4(s) + 6Cl(g) 4PCl3(g) P4(s) + 50(g) P4O10(S) PC13(g) + Cl(g) PCls
Question:
Given the following data: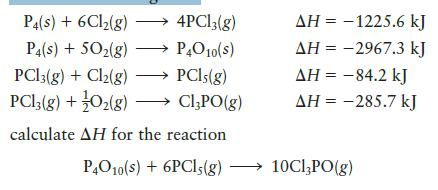
Transcribed Image Text:
P4(s) + 6Cl₂(g) →→→ 4PCl3(g) P4(s) + 50₂(g) P4O10(S) PC13(g) + Cl₂(g) PCls (g) PC13(g) + O₂(g) Cl₂PO(g) calculate AH for the reaction P4010(s) + 6PC15(g) AH = -1225.6 kJ AH = -2967.3 kJ AH = -84.2 kJ AH = -285.7 kJ 10Cl₂PO(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (13 reviews)
Given data Eq1 P4s 6Cl2g 4PCl3 H12256 KJ Eq2 P4s 5O2g P4O10s H29673 KJ ...View the full answer

Answered By

Godswill Okorie
M.Sc chemistry specialization in organic chemistry, B.ed .I am having industry experience of seven years working with Ranbaxy nd Shimadzu analytical India by working as an application chemistry.I am having good practical experience on chromatography techniques,which later helped me in my teaching.I worked as PGT chemistry teacher with KV and APS.
As a teacher I was able to achieve good results with my students.I used to take 11th and 12th chemistry and science to classes 7th ,8th and ninth. While teaching I used to guide students for various carrier opportunities.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Given the following data for a two-way ANOVA, identify the sets of null and alternative hypotheses, then use the 0.05 level in testing each null hypothesis. (Use data file XR12071.) Factor B 2 19 15...
-
Given the following data for a two-way ANOVA, identify the sets of null and alternative hypotheses, then use the 0.05 level in testing each null hypothesis. (Use data file XR12072.) Factor B 2 158...
-
Given the following data for a two-way ANOVA, identify the sets of null and alternative hypotheses, then use the 0.05 level in testing each null hypothesis. (Use data file XR12073.) Factor B 2 1...
-
A 200 g mass attached to a horizontal spring oscillates at a frequency of 1.5 Hz. At one instant, the mass is at x = 70 mm and has vx = -0.2 m/s. Determine (a) The period (b) The amplitude (c) The...
-
A random sample of 100 hospital patients suffering from depression received a particular treatment over a period of three months. Prior to the beginning of the treatment, each patient was classified...
-
Firemaster BBQ produces stainless steel propane gas grills. The company has been in operation for three years, and sales have declined each year due to increased competition. The following...
-
What constitutes the most significant conflict in the franchise system? What is the term used in lodging? In foodservice?
-
To the dismay of business travelers, airlines now discretely cater to families with young children who fly in first class (Katherine Rosman, Frequent Criers, Wall Street Journal, May 20, 2005, W1)....
-
Billings Company has the following information available for September 2 0 2 2 . ( a ) Compute the unit contribution margin. Unit contribution margin eTextbook and Media
-
Knockoffs Unlimited, a nationwide distributor of low-cost imitation designer necklaces, has an exclusive franchise on the distribution of the necklaces, and sales have grown so rapidly over the past...
-
Nitromethane, CH3NO2, can be used as a fuel. When the liquid is burned, the (unbalanced) reaction is mainly a. The standard enthalpy change of reaction (DH 8 rxn) for the balanced reaction (with...
-
Given the following data: FeO3(s) + 3CO(g) 3Fe2O3(s) + CO(g) 2Fe3O4(s) + CO2(g) Fe3O4(s) + CO(g) 3FeO(s) + CO(g) calculate AH for the reaction FeO(s) + CO(g) 2Fe(s) + 3CO(g) AH = -23 kJ AH = -39 kJ ...
-
If an integer expression appears in a Boolean context, how is its Boolean value determined?
-
Use the information below to answer the next question. Below are different graphs that could represent the magnitude of an Electric Field from a source. Teza E Distance E 4 Tza E Taza 2 Distance 5 3...
-
Factor out the GCF: 36c5 +54c8
-
Demonstrate that a circle with a radius of r has a circumference of 2 pi ( r ) . HINT: Begin by examining the equation for the upper semicircle, utilize the arc length formula, and then double the...
-
Graph the function f(x) = 3.x - 7.
-
Vine plc. produces a single product. The following information on inventory, purchases, and sales are available for the month of January 2018. DATE TRANSACTION NUMBER OF UNITS UNIT COST...
-
Suppose your favorite FM radio station broadcasts at a frequency of 101 MHz. What is the wavelength of this signal?
-
You work as an operations consultant for a textile company. Your client has a well-established distribution system in the US market. The company has hundreds of stores and four distribution centers....
-
The successive ionization energies for an unknown element are I1 = 896 kJ/mol I2 = 1752 kJ/mol I3 = 14,807 kJ/mol I4 = 17,948 kJ/mol To which family in the periodic table does the unknown element...
-
An unknown element is a nonmetal and has a valence electron configuration of ns2np4. a. How many valence electrons does this element have? b. What are some possible identities for this element? c....
-
An ion having a 41 charge and a mass of 49.9 amu has two electrons with n = 1, eight electrons with n = 2, and ten electrons with n = 3. Supply the following properties for the ion. (Hint: In forming...
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


