Identify the chiral carbon atoms in each of the following compounds: (a) Camphor, used in cooling salves,
Question:
Identify the chiral carbon atoms in each of the following compounds:
(a) Camphor, used in cooling salves,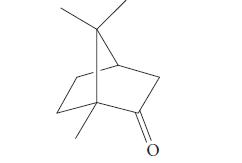
(b) Testosterone, a male sex hormone,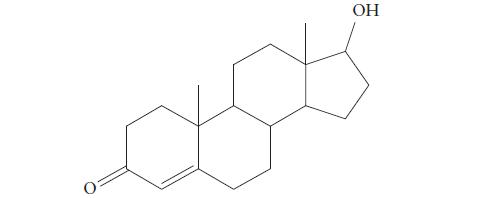
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (6 reviews)
An asterisk d...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Identify the chiral carbon atoms in each of the following compounds: (a) Menthol, the flavor of peppermint, (b) Estradiol, a female sex hormone, HO
-
(a) Draw the structure of 3,4,6-trimethyl-1-heptene. (b) Identify the chiral carbon atoms in the structure with stars. (c) Are cis and trans isomers possible for this molecule?
-
A sample of the male sex hormone testosterone, C19H28O2, contains 3.88 1021 hydrogen atoms. (a) How many atoms of carbon does it contain? (b) How many molecules of testosterone does it contain? (c)...
-
On July 7, Splish Ltd. purchased 1,100 common shares in a privately-owned company named TWR Ltd. As the TWR shares were not traded on any stock exchange, Splish elected to account for the investment...
-
Find the Thevenin's equivalent for the network in figure at terminals A-B. A -j1 a: 21,+ jia
-
Then how can the appeal court reverse? Isnt the court diminishing the defendants First Amendment rights?
-
Identify and discuss some decision-making situations in government when cost (expense) comparison is useful.
-
Zeus Computer Chips, Inc., used to have major contracts to produce the Centrino- type chips. The market has been declining during the past three years because of the quad- core chips, which it cannot...
-
last year inflation was 5.6 if your stock account earned
-
A branched hydrocarbon C 6 H 14 reacts with chlorine in the presence of light to give only two structural isomers with the formula C 6 H 13 Cl. Write the structural formulas of (a) The hydrocarbon;...
-
Which of the following molecules or ions may function as a nucleophile in a nucleophilic substitution reaction: (a) OH ; (b) NH 4 + ; (c) NH 2 2; (d) H 2 O?
-
A rocket sled exerts 3.00 10 4 N of thrust and has a mass of 2.00 10 3 kg. In how much time does it do zero to sixty? How many gs (see Exercise 14) does it achieve? Assume that on the surface of...
-
I need help with discussion posts that respond to 3 of these comments. 2 of them being the first on each picture. RUBRIC: articles to mention Coleman, R., & Banning, S. (2006). Network TV news'...
-
2. Best Use of Scarce Resource DigiCom Corporation produces three sizes of television sets: 12-inch screen, 26-inch screen, and 40-inch screen. Revenue and cost information per unit for each product...
-
Gunther invested $15,000 into a segregated fund with a 65% maturity guarantee 10 years ago. The fund is now maturing and has a current market value of $22,261. Gunther decides to withdraw his...
-
(a) Consider the following financial data (in millions of dollars) for Costello Laboratories over the period of 2014-2018: Year Sales Net income Total assets Common equity 2014 $3,800 $500 $3,900...
-
The Pizza Pie 'N Go sells about 2300 one-topping pizzas each month. The circle graph displays the most requested one-topping pizzas, by percentage, for one month. Most Popular One-Topping Pizzas...
-
To evaluate the equilibrium constant in Equation 6-2, we must express concentrations of solutes in mol/L, gases in bars, and omit solids, liquids, and solvents. Explain why.
-
The maximum pressure that can be developed for a certain fluid power cylinder is 15.0 MPa. Compute the required diameter for the piston if the cylinder must exert a force of 30 kN.
-
Draw the product obtained when each of the following compounds is treated with acetic anhydride in the presence of pyridine: (a) -d-Galactopyranose (b) -d-Glucopyranose (c) -d-Galactopyranose
-
Draw the product obtained when each of the compounds from the previous problem is treated with methyl iodide in the presence of silver oxide (Ag 2 O). In previous problem (a) -d-Galactopyranose (b)...
-
When -d-galactopyranose is treated with ethanol in the presence of an acid catalyst, such as HCl, two products are formed. Draw both products, and account for their formation with a mechanism.
-
Carla Vista Cart Inc. has the following information for 2026 : The rate of return on assets Carla Vista Cart Inc. is 81.06%17.27%30.58%14.00%
-
A man wishes to borrow 100$ for two years using the sinking fund method. He pays interest annually, at an annual effective interest rate of 5%. Construct a sinking fund schedule if he replaces the...
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...

Study smarter with the SolutionInn App


