In the spectrum of atomic hydrogen, several lines are generally classified together as belonging to a series
Question:
In the spectrum of atomic hydrogen, several lines are generally classified together as belonging to a series (for example, Balmer series or Lyman series, as shown in Fig. 1A.10). What is common to the lines within a series that makes grouping them together logical?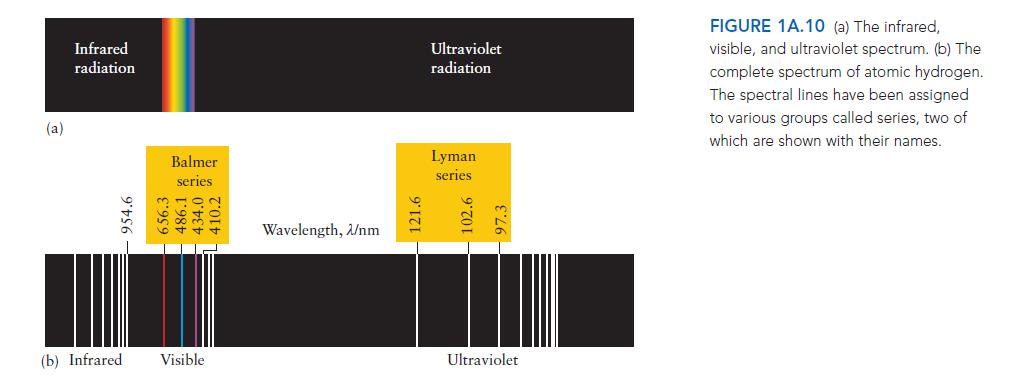
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





