Indium arsenide crystallizes in the zinc-blende ( sphalerite) structure (Fig. 3H.32). (a) What are the coordination numbers
Question:
Indium arsenide crystallizes in the zinc-blende ( sphalerite) structure (Fig. 3H.32).
(a) What are the coordination numbers of the indium and arsenide ions?
(b) What is the formula of indium arsenide?
FIGURE 3H.32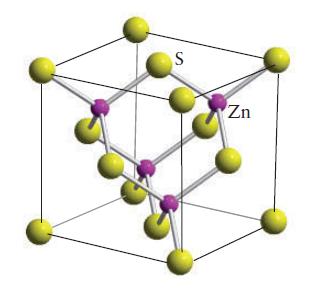
Transcribed Image Text:
S Zn
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)
a Indium arseni...View the full answer

Answered By

Akshay Singla
as a qualified engineering expert i am able to offer you my extensive knowledge with real solutions in regards to planning and practices in this field. i am able to assist you from the beginning of your projects, quizzes, exams, reports, etc. i provide detailed and accurate solutions.
i have solved many difficult problems and their results are extremely good and satisfactory.
i am an expert who can provide assistance in task of all topics from basic level to advance research level. i am working as a part time lecturer at university level in renowned institute. i usually design the coursework in my specified topics. i have an experience of more than 5 years in research.
i have been awarded with the state awards in doing research in the fields of science and technology.
recently i have built the prototype of a plane which is carefully made after analyzing all the laws and principles involved in flying and its function.
1. bachelor of technology in mechanical engineering from indian institute of technology (iit)
2. award of excellence in completing course in autocad, engineering drawing, report writing, etc
4.70+
48+ Reviews
56+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Silver iodide crystallizes in the zinc blende structure. The separation between nearest-neighbor cations and anions is approximately 325 pm, and the melting point is 558 C. Cesium chloride, by...
-
Copper iodide crystallizes in the zinc blende structure. The separation between nearest-neighbor cations and anions is approximately 311 pm, and the melting point is 606 C. Potassium chloride, by...
-
Which compound is most likely to crystallize in the zinc blende structure? (a) RbCl (Rb + radius = 148 pm; Cl radius = 181 pm) (b) MgCl 2 (Mg 2+ radius = 65 pm; Cl radius = 181 pm) (c) CuI (Cu +...
-
For the following exercises, use shells to find the volume generated by rotating the regions between the given curve and y = 0 around the x-axis. 130. y = 1-x,x = 0, and x = 1 131. y = x, x = 0, and...
-
Two couples act on the frame. If d = 4 ft, determine the resultant couple moment. Compute the result by resolving each force into x and y components and (a) Finding the moment of each couple (Eq....
-
The histogram displays the percent of foreign-born residents in each of the 50 states.12 Explain why this distribution of the percent of foreign born residents in the states is not approximately...
-
1. Suppose you have a project that will produce a single widget. Widgets today cost \($1\) and the project costs \($0.90\). The risk-free rate is 5%. Under what circumstances would you invest...
-
Develop a multiple-regression model for auto sales as a function of population and household income from the following data for 10 metropolitan areas: a. Estimate values for bt). b, and h: for the...
-
19. Harta Bhd's financial statements for the year to 30 June 2020 show a pre-tax profit of RM500,000. This is after charging depreciation of RM100,000. Depreciation for the year for tax purposes is...
-
Air bags in automobiles contain sodium azide, NaN 3 , which decomposes rapidly during a collision to give nitrogen gas and sodium metal. The nitrogen gas liberated by this process instantly inflates...
-
The effect of high pressure on organisms, including humans, is studied to gain information about deep-sea diving and anesthesia. A sample of air occupied 1.00 L at 25C and 1.00 atm. What pressure (in...
-
For the following exercises, determine whether the relation represents a function. {(1, 1),(2, 2),(3, 3)}
-
Use a substitution of the form u= ax + b to evaluate the following indefinite integral. S3x 3x+4 dx
-
Task 3 In order to support other staff to complete future risk assessments, produce a short-written report that explains. how hazards that become risks can be controlled the importance of fully...
-
let arr = [x => x + 5, x => 8, x => x * 2]; let b = X; let a = arr.reduce((acc, f) => acc + f(b), 0); If we know a is 28, what's the value of X?
-
2. (10 pts.) Identify the point symmetry elements of the structures for which the given directions are equivalent. Enumerate the elements (i.e., the individual symmetry operations) that make up the...
-
Theory Newton's second law can be written in a more general form as where is the momentum of system of N objects and is the net external force on the system. This relationship says that the rate at...
-
Use the identity for cos (A - B) and the identities And to prove that sin (A + B) = sin A cos B + cos A sin B A-cos(-A) cos A = sin(5-4)
-
Identify the most stable compound:
-
Calculate Ksp for iron(II) sulfide given the following data: Es. cell
-
Consider the galvanic cell based on the following halfreactions: b. Calculate ÎGo and K for the cell reaction at 25oC. c. Calculate cell at 25oC when [Au3+] = 1.0 Ã 102 M and [Tl+] = 1.0...
-
Consider the galvanic cell based on the following halfreactions: b. Calculate ÎGo and K for the cell reaction at 25oC. c. Calculate cell at 25oC when [Au3+] = 1.0 Ã 102 M and [Tl+] = 1.0...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Brief Exercise 10-6 Flint Inc. purchased land, building, and equipment from Laguna Corporation for a cash payment of $327,600. The estimated fair values of the assets are land $62,400, building...
-
"faithful respresentation" is the overriding principle that should be followed in ones prepaparation of IFRS-based financial statement. what is it? explain it fully quoting IAS. how this this...

Study smarter with the SolutionInn App


