Name the following compounds. a. b. c. (mathrm{HCOOH}) Cl- || C-OH
Question:
Name the following compounds.
a.
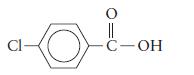
b.
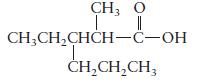
c. \(\mathrm{HCOOH}\)
Transcribed Image Text:
Cl- || C-OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Youve provided three compounds to name one as a text formula c and two as structural diagrams a and ...View the full answer

Answered By

HABIBULLAH HABIBULLAH
I have been tutor on chegg for approx 5 months and had solved a lot of questions.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Name the following compounds by IUPAC rules: a. b. H-C CH,CH-CH
-
Name the following compounds by the IUPAC system: a. CH3CH=C(CH2CH2CH3)2 b. (CH3)2CHCH"CHCH3 c. g. CH3-C-C-CH-CH, h. k.
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
You have a net income of $40 000 per year. Your expenses include the following: Rent: $800 per month Insurance: $225 per semi-annually Car Payment: $315 per month Car Expenses: $1 000 per year ...
-
Evaluate the integral and interpret it as a difference of areas. Illustrate with a sketch. Sm/2 48. 47. 'a sin x dx Ja/4
-
Explain how the accounting treatment of share dividends differs from that of cash dividends
-
Define the term predetermined and standard cost.
-
A fan that can provide air speeds up to 50 m/s is to be used in a low-speed wind tunnel with atmospheric air at 25C. If one wishes to use the wind tunnel to study flat-plate boundary layer behavior...
-
Question 4 3 pts On 1 January 2015, a company which prepares financial statements to 31 December each year acquires a machine on a finance lease. The fair value of the machine on 1 January 2015 is...
-
Salicylic acid has the following structure: Since salicylic acid has both an alcohol functional group and a carboxylic acid functional group, it can undergo two different esterification reactions...
-
Minoxidil \(\left(\mathrm{C}_{9} \mathrm{H}_{15} \mathrm{~N}_{5} \mathrm{O}ight)\) is a compound produced by the Pharmacia \& Upjohn Company that has been approved as a treatment for some types of...
-
Name the bases used for apportioning of selling and distribution (fi xed) overheads.
-
Reflect on your semester. How do you plan onmeasuringyour professionalgrowth in the future? What were the most challenging topics to you? What topics felt more intuitive/easy? How do you plan on...
-
Aside from shareholders, who do you believe is the second stakeholder in whose interests the company should be concerned? Justify your response What will you do to ensure the company's success...
-
a) What CSR did your organization do - how did it improve your organization's image? b) If your organization did not do any CSR, as the boss, what CSR activities would you suggest doing and why?
-
Do you believe NIL promotes "love of the game," or does it make college sports more about money and business? What are the most significant positive and negative effects of NIL, in your opinion? What...
-
Even well-managed organizations do not always work as efficiently and effectively as management would like. At Hewlett-Packard (HP), billions of dollars of product are being shipped - from computers...
-
Use the quadratic approximation to estimate the following values. Compare the estimates with the exact answer. 1. 2.023 2. 3.032 3. 4.01 4. 6 5. sin(0.02) 6. cos(-0.02)
-
If your school has a subscription to the FASB Codification, go to aaahq.org/ ascLogin.cfm to log in and prepare responses to the following. (a) What is the stock dividend? (b) What is a stock split?...
-
Describe the structure of the formaldehyde molecule, CH 2 O, in terms of hybrid orbitals, bond angles, and - and -bonds. The C atom is the central atom to which the other three atoms are attached.
-
Noting that the bond angle of an sp 3 hybridized atom is 109.5 and that of an sp 2 hybridized atom is 120, do you expect the bond angle between two hybrid orbitals to increase or decrease as the...
-
Draw the complete Lewis structure for each of the following compounds: (a) Ammonium chloride; (b) Potassium phosphide; (c) Sodium hypochlorite.
-
Phantom Consulting Inc. is a small computer consulting business. The company is organized as a corporation and provides consulting services, computer system installations, and custom program...
-
Sam owns a 25% in Spade, LLC. In 2021, Spade reports $100,000 or ordinary income. What is Sams qualified business income (QBI) deduction? answer is 5,000 but please show how to get it
-
crane Inc. common chairs currently sell for $30 each. The firms management believes that it's share should really sell for $54 each. If the firm just paid an annual dividend of two dollars per share...

Study smarter with the SolutionInn App


