One structure of the octahedral complex FeCl 2 (NH 3 ) 3 SCN is shown below. Draw
Question:
One structure of the octahedral complex FeCl2(NH3)3SCN is shown below. Draw all possible isomers of this complex.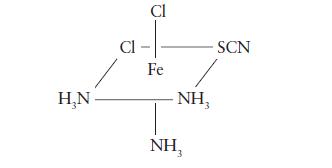
Transcribed Image Text:
HN Cl CI Fe - SCN NH 3 NH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
There are a total of six isomers of FeNH 3 3 Cl 2 S...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Draw all possible isomers for the molecule C3H5Br.
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
How many isomers are possible for an octahedral complex of general formula [MA 2 BCDE]? Draw all that are possible.
-
Two wires carrying equal and opposite currents are twisted together in the construction of a circuit. Why does this technique reduce stray magnetic fields? Please explain for dummies.
-
Write a program to merge two sorted files into a single file using any version of Windows.
-
What is the composition, in atom percent, of an alloy that contains 98 g tin and 65 g of lead?
-
The pupil of the eye (Fig. Q26.19) defines the aperture of the eye and thus determines how much light enters the eye and strikes the retina. If the pupil opens up, does the f-number of the eye...
-
The following are typical questions that might appear on an internal control questionnaire for payroll activities: 1. Is there adequate separation of duties between employees who maintain human...
-
USE EXCEL PLEASE 5. According to College Board, national average SAT score (Math section) in 2017 has been 500 with standard deviation of 102. We took a random sample of 50 students who took SAT...
-
The complex [Co(CN) 6 ] 3 is pale yellow. (a) How many unpaired electrons are present in the complex? (b) If ammonia molecules replace the cyanide ions as ligands, will the wavelength of the...
-
cis-Platin is an anticancer drug with a three-dimensional structure that can be viewed on the Internet. (a) What are the formula and systematic name for the compound cis-platin? (b) Draw any isomers...
-
Show that curvature at an inflection point of a plane curve y = (x) is zero.
-
Draw a bar graph for each data set in Problems 32-35. Data set \(\mathrm{D}\) Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 35 25 25 16 14 1...
-
Draw a line graph for each data set in Problems 36-39. Data set A Data set A: The annual wages of employees at a small accounting firm are given in thousands of dollars. 25 16 25 25 14 18 1 2 2 2...
-
For each of the angles shown: (i) Estimate its size (ii) Measure it and check how good your estimate was. Aim for your estimate to be within 10 of the actual angle. a. b. c. d. e. f.
-
For the quasispin model of Problem 31.1 , find the eigenvalues of $s_{0}^{(m)}$ for the levels labeled by $m$. Show that the system has a total quasispin $S$ that is the vector sum of quasispins for...
-
A sole proprietorship was started on January 1, 2005, when it received \($60,000\) cash from Mark Pruitt, the owner. During 2005, the company earned \($40,000\) in cash revenues and paid \($19,300\)...
-
Use APPLE.RAW to verify some of the claims made in Section 6.3. (i) Run the regression ecolbs on ecoprc, regprc and report the results in the usual form, including the R-squared and adjusted...
-
Gopher, Inc. developing its upcoming budgeted Costs of Quality (COQ) with the following information: Expense Item Budget Raw Materials Inspection $ 15,000 EPA Fine 200,000 Design Engineering 15,000...
-
How does the viscosity of a gas vary with pressure?
-
What is the expression for the diffusion coefficient, D, in terms of gas kinetic theory parameters? How is D expected to vary with an increase in molecular mass or collisional cross section?
-
What is the general relationship between the spatial gradient in a system property and the flux of that property?
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


