Predict the standard cell potential and calculate the standard reaction Gibbs free energy for galvanic cells having
Question:
Predict the standard cell potential and calculate the standard reaction Gibbs free energy for galvanic cells having the following cell reactions: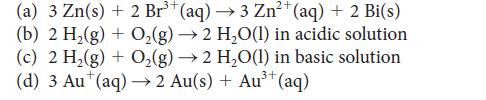
Transcribed Image Text:
(a) 3 Zn(s) + 2 Br³ (aq) →3 Zn²+ (aq) + 2 Bi(s) (b) 2 H₂(g) + O₂(g) → 2 H₂O(l) in acidic solution - (c) 2 H₂(g) + O₂(g) → 2 H₂O(1) in basic solution (d) 3 Au* (aq) → 2 Au(s) + Au³+ (aq) 3+
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Here are the predicted standard cell potentials and calculated standard reaction Gibbs free energies ...View the full answer

Answered By

Ann Wangechi
hey, there, paying attention to detail is one of my strong points, i do my very best combined with passion. i enjoy researching since the net is one of my favorite places to be and to learn. i am a proficient and versatile blog, article academic and research writing i possess excellent English writing skills, great proof-reading. i am a good communicator and always provide feedback in real time. i'm experienced in the writing field, competent in computing, essays, accounting and research work and also as a Database and Systems Administrator
4.90+
151+ Reviews
291+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The standard reduction potentials of the following half-reactions are given in Appendix E: (a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest...
-
Suppose that you are in an engineering competition in which commercial power sources are prohibited. You might decide to use a Daniell cell to power a model electric car. You will need to know the...
-
Fill in the blanks in the table below. The problem is a "puzzle" so the blanks are not necessarily filled in sequentially. Hint: Determine the total fixed cost first. Instructions: Round your answers...
-
You are looking at buying a piece of real estate and you intend to borrow as much as you possibly can from a bank to buy the property. The bank you are dealing with has a requirement that the LVR for...
-
Standard-costing with beginning and ending work in process. Paquitas Pearls Company (PPC) is a manufacturer of knock off jewelry. Paquita attends Fashion Week in New York City every September and...
-
Let p denote the probability of some event. Plot the amount of information gained by the occurrence of this for 0 < p < 1.
-
As it has been standardized, the response z vmail messages has a standard deviation of 1.0. What would be the typical error in predicting z vmail messages if we simply used the sample mean response...
-
Corporate bonds issued by Johnson Corporation currently yield 8 percent. Municipal bonds of equal risk currently yield 6 percent. At what tax rate would an investor be indifferent between these two...
-
Macmillan Learnin If you and your friend talk about many different toples in great deal, your sen-disclosure hus high breadth and high depth. O high breadth and low depth. O low breadth and high...
-
Given a network for an HR training project with normal times and crash times (in parentheses), find the cost-duration history. Assume indirect costs for facilities and equipment are $100 per day. The...
-
Dinitrogen monoxide, N 2 O, reacts with water to form hyponitrous acid, H 2 N 2 O 2 (aq), in a Lewis acidbase reaction. (a) Write the chemical equation for the reaction. (b) Draw the Lewis structures...
-
Calculate the pH and pOH of each of the following aqueous solutions of strong acid or base: (a) 0.0356 m HI(aq); (b) 0.0725 m HCl(aq); (c) 3.46 * 10 3 m Ba(OH) 2 (aq); (d) 10.9 mg of KOH dissolved...
-
Discuss what it means to practice good business ethics, and highlight three factors that influence ethical decision making.
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
A medical researcher in India obtained blood specimens from 31 young children, all of whom were infected with malaria. The following data, listed in increasing order, are the numbers of malarial...
-
The ultimate goal of Google, Bing, and other consumer search engines is to provide users with search listings that contain useful information on the topic of their search. What recommendations would...
-
When the following compound is treated with excess methyl iodide, a quaternary ammonium salt is obtained that bears only one positive charge. Draw the structure of the quaternary ammonium salt. *NH2
-
Draw a mechanism for the following transformation: 'CI Z Z
-
In Problem 22.38, we saw an intramolecular example of a malonic ester synthesis using excess base and 1,4-dibromobutane. If this dibromide is used in an acetoacetic ester synthesis, an intramolecular...
-
nformation pertaining to Noskey Corporation s sales revenue follows: November 2 0 2 1 ( Actual ) December 2 0 2 1 ( Budgeted ) January 2 0 2 2 ( Budgeted ) Cash sales $ 1 0 5 , 0 0 0 $ 1 1 5 , 0 0 0...
-
The management team of Netflix maintains a stable dividend using the Lintner model: Dt+1 = Dt + EPS Target Payout Where Dt (Dt+1) = dividend in the current period t (the next period t + 1) EPSt =...
-
#1 #2 hapter 50 10 D Werences lav Help Required information [The following information applies to the questions displayed below) Archer Company is a wholesaler of custom-built air-conditioning units...

Study smarter with the SolutionInn App


