The electrical and thermal conductivities of the Group 11 and Group 12 elements, along with manganese, were
Question:
The electrical and thermal conductivities of the Group 11 and Group 12 elements, along with manganese, were measured (see table). Use the data to answer the following:
(a) The electrical conductivity of a metal is related to the ability of electrons to move freely through the lattice. Can the same be said of the thermal conductivity? Justify your answer by plotting the thermal conductivity against electrical conductivity.
(b) Explain why the electrical and thermal conductivities of Group 11 metals are high while those of Group 12 metals are low.
(c) Explain why the electrical and thermal conductivities of manganese are so low.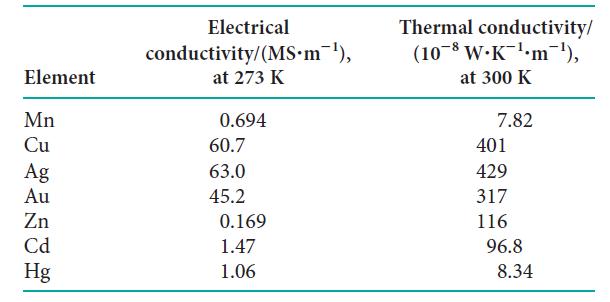
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:





