The flask below contains atoms of A (red) and B (yellow). They react as follows 2 A
Question:
The flask below contains atoms of A (red) and B (yellow). They react as follows 2 A (g) + B (g) → A2B(g), with K = 0.25. Draw a picture of the flask and its contents after the reaction has reached equilibrium.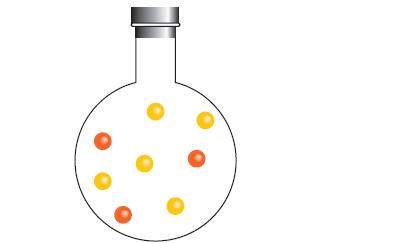
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Vapor pressure increases because of the increased ...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
For the following two graphics, provide the specified information below for each. Inverse Demand: P= 43.75 - .00625 Q; MR = 43.75 - 0.0125 Q 25 20 15 $ per unit 10 10 5 0 MC 500 1000 1500 ATC 2000 -...
-
Suppose you are designing a chemical plant that is providing phosphorus compounds to other industries and you need to explore the equilibrium properties for the reaction of PCl 5 (g) PCl 3 (g) + Cl...
-
The following picture represents atoms of hypothetical, nonmetallic, monatomic elements A, B, and C in a container at a temperature of 4 K (the piston maintains the pressure at 1 atm). None of these...
-
Find the coordinates of the midpoint of the segment with the given endpoints. P(4, -1) and Q(-2, 3)
-
Which of the following K-value expressions, if any, is (are) rigorous? For those expressions that are not rigorous, cite the assumptions involved. (a) Ki = ФiL/ФiV (b) Ki =...
-
A heat engine operating between 200C and 80.0C achieves 20.0% of the maximum possible efficiency. What energy input will enable the engine to perform 10.0 kJ of work?
-
As auditor for Banquo & Associates, you have been assigned to check Duncan Corporations computation of earnings per share for the current year. The controller, Mac Beth, has supplied you with the...
-
Kerwin Industries is deciding whether to automate one phase of its production process. The manufacturing equipment has a six- year life and will cost $ 925,000. Projected net cash inflows are as...
-
en 25 At the breakeven point 1 red d out of a question Select one: O a Sales will be equal to variable costs minus fixed costs O b. Sales will be equal to variable costs plus target profit O c Fixed...
-
How can a nurse aide identify Mr. James’s medical problems, his emotional and physical needs, and deal with these problems? Pay close attention to safety issues specific for an individual who...
-
Calculate the solubility in water (in milligrams per liter) of (a) Air at 0.80 atm; (b) He at 0.80 atm; (c) He at 36 kPa. The temperature is 20C in each case, and the pressures are partial pressures...
-
The vapor pressure of benzene is 100.0 Torr at 26 C. A nonvolatile compound was added to 0.400 mol C 6 H 6 (l) at 26C and the vapor pressure of the benzene in the solution decreased to 68.0 Torr....
-
Which of the following is unlikely to be a concern of internal auditors when they evaluate how well designers undertook the design and acquisition of the hardware/system software platform to be used...
-
Financial strength can be defined as the capacity to produce enough cash flows and earnings to pay creditors, investors, and other debts, as well as to cover expenses. Even though sales by themselves...
-
The RMS Titanic was the most technologically advanced liner in the world in the year 1912. At 11:40pm or Sunday, April 14 of that year, the Titanic struck an iceberg and sank in less than three...
-
1. A management consultant is hired by a manufacturing firm to determine the best site for its next production facility. The consultant has had several meetings with the company's senior executives...
-
The figure below shows that a pump is used to transfer water from a reservoir at ground level to a storage take that is elevated. The pump is located 10 ft above the water surface of the reservoir...
-
P6-3 (Algo) Comparing and Contrasting the Effects of Inventory Costing Methods on Financial Statement Elements LO6-2, 6-3 Neverstop Corporation sells item A as part of its product line. Information...
-
What other recovery options does Recuva come with?
-
Define deferred revenue. Why is it a liability?
-
Draw resonance structures for each of the following anions. a. b. c.
-
In the reversible adiabatic expansion of 1.75 mol of an ideal gas from an initial temperature of 27.0C, the work done on the surroundings is 1300. J. If C V ,m = 3/2R, calculate q, w, U, and H.
-
For a given set of conditions, the fugacity of a gas is greater than the pressure. What does this tell you about the interaction between the molecules of the gas?
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


