Question: The following data were collected for a gas sample consisting of 5.00 mol of molecules in a rigid container. (a) Determine the volume of the
The following data were collected for a gas sample consisting of 5.00 mol of molecules in a rigid container.
(a) Determine the volume of the sample.
(b) Plot the data either by hand or using a spreadsheet. Now suppose you add another 5.00 mol of gas molecules into the same volume. Plot the corresponding line for the second sample.
(c) At what temperature (in kelvins) do the two lines intersect?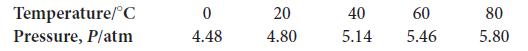
Temperature/C Pressure, P/atm 0 4.48 20 4.80 40 5.14 60 5.46 80 5.80
Step by Step Solution
3.37 Rating (172 Votes )
There are 3 Steps involved in it
a The volume can be calculated using any set of data in the ta... View full answer

Get step-by-step solutions from verified subject matter experts


