The following rate data were collected for the reaction 2 A(g) + 2 B(g) + C(g)
Question:
The following rate data were collected for the reaction 2 A(g) + 2 B(g) + C(g) → 3 G(g) + 4 F(g):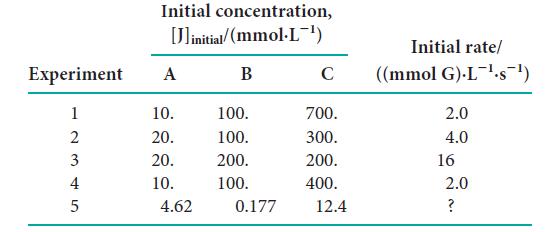 (a) What is the order for each reactant and the overall order of the reaction?
(a) What is the order for each reactant and the overall order of the reaction?
(b) Write the rate law for the reaction.
(c) Determine the reaction rate constant.
(d) Predict the initial rate for Experiment 5.
Transcribed Image Text:
Initial concentration, [linitial/(mmol.L-¹) B Experiment A 12345 10. 20. 20. 10. 4.62 100. 100. 200. 100. 0.177 C 700. 300. 200. 400. 12.4 Initial rate/ ((mmol G).L¹-s¯¹) 2.0 4.0 16 2.0 ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
a first order with respect to ...View the full answer

Answered By

Caroline Kinuthia
Taking care of the smaller details in life has a larger impact in our general well being, and that is what i believe in. My name is Carol. Writing is my passion. To me, doing a task is one thing, and delivering results from the task is another thing. I am a perfectionist who always take things seriously and deliver to the best of my knowledge.
4.90+
1934+ Reviews
4277+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
The outer and inner wheels are spun. Assuming that the wheels are independent and the outcomes are equally likely, determine the probability of obtaining. Numbers greater than 5 on both wheels. 3 2 4...
-
Use the pattern below. Each figure is made of squares that are 1 unit by 1 unit. a. Find the distance around each figure. Organize your results in a table. b. Use your table to describe a pattern in...
-
In Exercises delete part of the domain so that the function that remains is one-to-one. Find the inverse function of the remaining function and give the domain of the inverse function. f(x) = (x - 3)...
-
Determine the degrees of freedom under the following conditions: (a) Tl-20 wt% Pb at 325 C and 400 C; (b) Tl-40 wt% Pb at 325 C and 400 C; (c) Tl-90 wt% Pb at 325 C and 400 C. Refer to the phase...
-
Here is a bogus About me description. Which of the following passwords could be guessed from the About Me? a. Walker b. jebBrother c. IBeat Al d. 1600Penn-Ave.NW e. texas
-
Non-uniform ball in Figure, a ball of mass M and radius R rolls smoothly from rest down a ramp and onto a circular loop of radius 0.48 m. The initial height of the ball is h = 0.36 m. At the loop...
-
1. Under the equity method, Pam reports investment income from Sun for 2016 of: a $120 b $96 c $80 d $104 loss
-
The comparative balance sheet of Whitman Co. at December 31, 2016 and 2015, is as follows: The noncurrent asset, noncurrent liability, and stockholders' equity accounts for 2016 are as follows: Dec....
-
You are analyzing the sale of an asset. The asset has a basis of $24,000 and was depreciated straight-line to $3,000 salvage over 7 years. The asset will be sold after 9 years. The book value of the...
-
The issue had come up again and again in various management meetings and company seminars. Novartis had too many products and needed to reduce the product proliferation that had occurred. Thomas...
-
Sulfuryl chloride, SO 2 Cl 2 , decomposes by first-order kinetics, and k r = 2.81 * 10 3 min 1 at a certain temperature. (a) Determine the half-life for the reaction. (b) Determine the time needed...
-
Repeat Exercise 7C.13 for the same reaction taken to be second order in each direction. Exercise 7C.13 Consider the reaction A B, which is first order in each direction with rate constants k r and k...
-
Normal and abnormal spoilage in units. The following data, in physical units, describe a grinding process for January: Inspection occurs at the 100% completion stage. Normal spoilage is 5% of the...
-
In this Capstone experience, you will develop a strategy playbook for a selected organization. You may be familiar with the concept of a playbook as it relates to a sports team, but what might that...
-
On January 1, 2024, the general ledger of Big Blast Fireworks includes the following account balances: Accounts Cash Debit Credit $25,900 Accounts Receivable 46,500 Allowance for Uncollectible...
-
The WRX can travel 1 / 4 of a mile in 1 3 . 9 sec . Calculate the acceleration over this distance if assumed constant.
-
You are expected to develop two simulators mimicking the behavior and analyze the performance of iterative multiplication algorithm and add-and-shift multiplication algorithm. You are free to use any...
-
A.BRK Company, which Manufactures bags, has a Capacity of 130,000 bags per month. Currently its operating capacity is 100,000 units. The company receives a special order of 20,000 bags at $9 a bag. A...
-
A researcher is planning to conduct an experiment to compare two treatments in which matched pairs of subjects will be given the treatments and a sign test will be used, with a non directional...
-
Draw two scatterplots, one for which r = 1 and a second for which r = 21.
-
You drew the two chair conformations of lindane. Carefully inspect them, and predict the difference in energy between them, if any.
-
Compound A exists predominantly in a chair conformation, while compound B exists predominantly in a twist boat conformation. Explain. Compound A Compound B
-
Draw Haworth projections for cis-1,3-dimethylcyclohexane and trans-1,3-dimethylcyclohexane. Then, for each compound, draw the two chair conformations. Use these conformations to determine whether the...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...
-
Assume that gasoline costs $ 3 . 2 0 per gallon and you plan to keep either car for six years. How many miles per year would you need to drive to make the decision to buy the hybrid worthwhile,...

Study smarter with the SolutionInn App


