The reaction [2 mathrm{NO}(g)+mathrm{Cl}_{2}(g) longrightarrow 2 mathrm{NOCl}(g)] was studied at (-10^{circ} mathrm{C}). The following results were obtained,
Question:
The reaction
\[2 \mathrm{NO}(g)+\mathrm{Cl}_{2}(g) \longrightarrow 2 \mathrm{NOCl}(g)\]
was studied at \(-10^{\circ} \mathrm{C}\). The following results were obtained, where
\[\text { Rate }=-\frac{d\left[\mathrm{Cl}_{2}ight]}{d t}\]
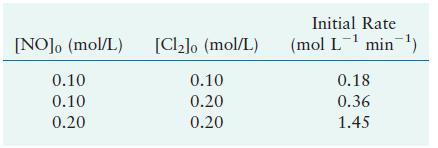
a. What is the rate law?
b. What is the value of the rate constant?
Transcribed Image Text:
[NO]o (mol/L) 0.10 0.10 0.20 [Cl]o (mol/L) 0.10 0.20 0.20 Initial Rate (mol L min) 0.18 0.36 1.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
To determine the rate law for the given reaction we need to analyze how changes in the concentrations of the reactants affect the rate of the reaction The rate law can be written in the general form Rate k NOx Cl2y where k is the rate constant NO and Cl2 are the concentrations of the reactants and x and y are the orders of the reaction with respect to NO and Cl2 respectively We will use the provided initial rates and concentrations to deduce the values of x and y Lets examine how the initial rate changes when the concentration ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The enzyme carboxypeptidases catalyses the hydrolysis of polypeptides and here we consider its inhibition. The following results were obtained when the rate of the enzymolysis of...
-
The following results were obtained at 600 K for the de-composition of ethanol on an alumina (Al2O3) surface C2H5OH(g) C2H4(g) + H2O(g) a. Predict PTotal in torr at t = 80. s. b. What is the value of...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Given an array, Arr[] of integer numbers have size N. The task is to find out single or consecutive numbers from Arr[] with 0 sum. When found, print "1" otherwise "0". Example-1: Input: 4 (6,-2,3,-1)...
-
A direction field for a differential equation is shown. Draw, with a ruler, the graphs of the Euler approximations to the solution curve that passes through the origin. Use step sizes h = 1 and h =...
-
You have just turned twenty-five years old, and are about to start working with no current assets or debt. Your current salary is w25 = $50,000 per year, payable at the end of the year. You expect...
-
For each of the following 99% confidence intervals for b1 in simple linear regression, decide whether there is evidence of a positive or negative linear relationship between y and x: LO9 a. (8, 80)...
-
Wyco Park, a public camping ground near the Four Corners National Recreation Area, has compiled the following financial information as of December 31, 2019. Instructions (a) Determine Wyco Park's net...
-
On February 20, 20X4 you are well into the field work of the 12/31/20X3 audit and the following issues have arisen during the audit of Beta Computer Equipment Company (BCE.) Service revenue Account...
-
The reaction \[2 \mathrm{I}^{-}(a q)+\mathrm{S}_{2} \mathrm{O}_{8}{ }^{2-}(a q) \longrightarrow \mathrm{I}_{2}(a q)+2 \mathrm{SO}_{4}{ }^{2-}(a q)\] was studied at \(25^{\circ} \mathrm{C}\). The...
-
The microwave spectrum of 1 H 35 Cl shows that the transition from J = 0 to J = 1 requires electromagnetic radiation with a wavelength of 4.85 10 4 m. Calculate the bond length of the 1 H 35 Cl...
-
In Exercises, find each indefinite integral. 3x+4x dx
-
According to a recent study, 21% of American college students graduate with no student loan debt. Suppose we obtain a random sample of 106 American college students and record whether or not they...
-
Differentiate the following with respect to x: a. y=5x+2x + x + 15 b. y=4x+3x - 4x - 10 c. y = 3Sin(5x) d. y = 3Cos(3x) e. y=10e -25x f. y = log(6x)
-
Question 2. The rate of drug destruction by the kidneys is proportional to the amount of the drug in the body. The constant of proportionality is denoted by K. At time t the quantity of the drug in...
-
5. 6. -1 (4a) U u X2 1 X2 -2 x -1 -2 12 (4b) U -2 2 Y y 16 x2 X2 3 1 (4c) U u - x 2 Y y -8 Y y -20 5 x X2 2 Find the state space models of the three systems shown in Fig. 4a, Fig. 4b, and Fig. 4c,...
-
Given the following data for Mehring Company, compute total manufacturing costs, prepare a cost of goods manufactured statement, and compute cost of goods sold. Direct materials used $230,000...
-
The equations in Exercise 16. Redraw the phase planes for the above problem, but make the other choice for the vertical variable. Check that you get the same equilibrium. Exercise 16 Competition...
-
Coastal Refining Company operates a refinery with a distillation capacity of 12,000 barrels per day. As a new member of Coastal's management team, you have been given the task of developing a...
-
Use the information in Fig. 2D.11 to estimate the length of (a) The CO bond in CO 2 ; (b) The CO and CN bonds in urea, OC(NH 2 ) 2 ; (c) The OCl bond in HClO; (d) The NCl bond in NOCl. CO B Bond...
-
Draw the Lewis structure and the VSEPR formula, list the shape, and predict the approximate bond angles of (a) PCl 3 F 2 ; (b) SnF4; (c) SnF 6 2- ; (d) IF 5 ; (e) XeO 4 .
-
Both NH 2 and NH 2 + are angular species, but the bond angle in NH 2 is less than that in NH 2 +. (a) What is the reason for this difference in bond angles? (b) Take the x -axis as lying...
-
1,600 Balance Sheet The following is a list (in random order) of KIP International Products Company's December 31, 2019, balance sheet accounts: Additional Paid-In Capital on Preferred Stock $2,000...
-
Question 3 4 pts 9 x + 3 x 9 if x 0 Find a) lim f(x), b) lim, f(x), C), lim , f(x) if they exist. 3 Edit View Insert Format Tools Table : 12pt M Paragraph B IV A2 Tv
-
Mr. Geoffrey Guo had a variety of transactions during the 2019 year. Determine the total taxable capital gains included in Mr. Guo's division B income. The transactions included: 1. On January 1,...

Study smarter with the SolutionInn App


