There are three isomers of difluoroethene, C 2 H 2 F 2 , which differ in the
Question:
There are three isomers of difluoroethene, C2H2F2, which differ in the locations of the fluorine atoms.
(a) Which of the forms are polar?
(b) Which has the largest dipole moment?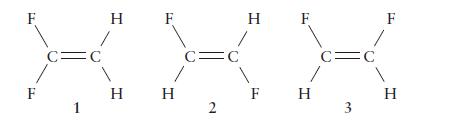
Transcribed Image Text:
F F C= 1 C O H F H H C= 2 H F F H C= 3 F H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a Polarity Isomer 1 transdifluoroethene Nonpolar In this isomer the CF bond dipoles cancel each othe...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
There are three isomers of dichlorobenzene, C 6 H 4 Cl 2 , which differ in the relative positions of the chlorine atoms on the benzene ring. (a) Which of the three forms are polar? (b) Which has the...
-
A student claims there are three isomers of propanol: 1-propanol, 2-propanol, and 3-propanol. Is he correct? Explain.
-
A molecule XF6 (having no lone pairs) has a dipole moment of zero. (X denotes an unidentified element.) When two atoms of fluorine have been taken away, you get the molecule XF4, which has dipole...
-
Consider the following independent situations found during audit testing of Faran Ltd, which has a balance date of 30 June 2019. Assume that all the situations are material. (i)Recent industrial...
-
Why should the factors under the control of the investment center manager (revenues, expenses, and invested assets) be considered in computing the rate of return on investment?
-
The figure shows a curve C given by a vector function r(t). (a) Draw the vectors r(4.5) r(4) and r(4.2) r(4). (b) Draw the vectors (c) Write expressions for r'(4) and the unit tangent vector T(4)....
-
Study of sex offenders. A study of sex offenders in the Canadian Federal Prison System was published in the British Journal of Criminology (May 2014). The following data were collected for each of 59...
-
Pelzer Company reconciled its bank and book statement balances of Cash on August 31 and showed two cheques outstanding at that time, #5888 for $6,220.00 and #5893 for $1,485.65. The following...
-
Alsup Consulting sometimes performs services for which it receives payment at the conclusion of the engagement, up to six months after services commence. Alsup recognizes service revenue for...
-
Draw Lewis structures for each of the following species and predict the hybridization at each carbon atom: (a) H 2 CCH ; (b) H 2 CCH 3 + ; (c) H 3 CCH 2 .
-
Explain why the lattice energy of lithium chloride (861 kJ mol -1 ) is greater than that of rubidium chloride (695 kJ mol -1 ), given that they have similar arrangements of ions in the crystal...
-
On September 1, 2017, Praise Corporation issued $600,000 of 10-year, 3% bonds at 96. Interest is payable semi-annually on September 1 and March 1. Praise's fiscal year end is February 28....
-
Find the unknown angle measures. 49 60 Drawing is not to scale. I = y = In S
-
Q5 For this question, use data from only restaurants with between 50 and 60 items in the data set. Predict total fat from cholesterol, total carbs, vitamin a, and restaurant. Remove any...
-
A meteorologist believes that there is a relationship between the daily mean windspeed, w kn, and the daily mean temperature, t C. A random sample of 9 consecutive days is taken from past records...
-
Suppose k(x) = f(g(h(x))). Given the table of values below, determine k' (1). g(x) h(x) f'(x) g'(x) h'(x) x f(x) 1 -6 -3 3 6 -6 -6 3 -3 4 1 -7 -2 5 4 -2 7 3 1 -7 -8
-
In a research study women with metastatic stomach cancer responded to the Symptom Distress Scale and the Profile of Mood States. A correlation coefficient was reported: r = 0.5, p = 0.03. How would...
-
What is wrong with the following procedure for obtaining magnesium? MgCO3 MgO(s) + CO2(g) MgO(s) + CO(g) Mg(s) + CO2(g)
-
An environmentalist wants to determine if the median amount of potassium (mg/L) in rainwater in Lincoln County, Nebraska, is different from that in the rainwater in Clarendon County, South Carolina....
-
The decomposition of N 2 O 5 in the gas phase was studied at constant temperature: The following results were collected: Using these data, verify that the rate law is first order in [N 2 O 5 ], and...
-
The reaction between bromate ions and bromide ions in acidic aqueous solution is given by the following equation: Table 15.5 gives the results of four experiments involving this reaction. Using these...
-
The compound \(\mathrm{NO}_{2} \mathrm{Cl}\) is thought to decompose to \(\mathrm{NO}_{2}\) and \(\mathrm{Cl}_{2}\) by the following mechanism: Derive the rate law for the production of...
-
Ted and his partners have contracted to purchase the franchise nights worth 561 000 to open and operate a specialty pizza restaurant called Popper with a renewable agrement, the partners have agreed...
-
Your answer is partially correct. Martin Company's chief financial officer feels that it is important to have data for the entire quarter especially since their financial forecasts indicate some...
-
Kellog Corporation is considering a capital budgeting project that would have a useful life of 4 years and would love testing 5156.000 in equipment that would have zeto salvage value at the end of...

Study smarter with the SolutionInn App


