To analyze the alcohol content of a certain wine, a chemist needs 1.00 L of an aqueous
Question:
To analyze the alcohol content of a certain wine, a chemist needs 1.00 L of an aqueous 0.200 M K2Cr2O7 (potassium dichromate) solution. How much solid K2Cr2O7 must be weighed out to make this solution?
Figure 4.9
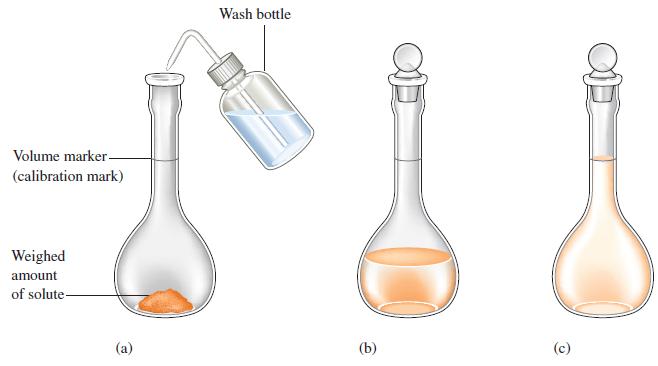
Transcribed Image Text:
Volume marker- (calibration mark) Weighed amount of solute- (a) Wash bottle (b) (c)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
First determine the moles of KCrO7 required 0200 mol KCrO7 L solution 100 L ...View the full answer

Answered By

Umber Talat
I am providing full time mentoring and tutoring services in Business Finance, Contemporary issue in Global Economy, Quantitative Techniques, Principles of Marketing, strategic marketing, International Marketing, Organizational Behavior (OB), Consumer Behavior, Sales Force Management, Strategic Brand Management, Services Marketing, Integrated Marketing Communication (IMC), Principles of Management, General Management, Strategic Management, Small and Medium Enterprise Management, Innovation Management, Change Management, Knowledge Management, Strategic Planning, Operations Management, Supply Chain Management, Logistics Management, Inventory management, Total Quality Management (TQM), Productions Management, Project Management, Production Planning, Human Resource Management (HRM), Human Resource Development, Strategic HRM, Organizational Planning, Performance and Compensation Management, Recruitment and Selection, Organizational Development, Global Issues in Human Resource Management, Retail Marketing, Entrepreneurship, Entrepreneurial Marketing, International Business, Research Methods in Business, Business Communication, Business Ethics.
4.70+
158+ Reviews
236+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
The energy content of a certain food is to be determined in a bomb calorimeter that contains 3 kg of water by burning a 2-g sample of it in the presence of 100 g of air in the reaction chamber. If...
-
What is the is the effect of abolition of an import quota on (i) national saving, (ii) domestic investment, (iii) NCO, (iv) the real exchange rate, and (v) net exports?
-
A rod is bent into a circular arc of radius 4 in. as shown. For the given loading, determine the internal forces at point J when 30 θ = °. 12 Ih B. 4 in.
-
Each member of a large genetics class grows 12 pea plants from an independent pea family. Each family is expected to have 3/4 plants with smooth peas and 1/4 of the plants with wrinkled peas. On...
-
5. What is goodwill? How is goodwill treated under current GAAP/IFRS?
-
Waldo Entertainment Products, Inc. is negotiating with Disney for the rights to manufacture and sell superhero-themed toys for a three-year period. At the end of year 3, Waldo plans to liquidate the...
-
he November 3 0 , 2 0 2 0 , unadjusted trial balance of Clear Choice is found in the Trial balance tab. Clear Choice had the following transactions and events in December 2 0 2 0 . December 2 Paid $...
-
Using the solubility rules in Table 4.1, predict what will happen when the following pairs of solutions are mixed. a. KNO 3 (aq) and BaCl 2 (aq) b. Na 2 SO 4 (aq) and Pb(NO 3 )2(aq) c. KOH(aq) and...
-
Typical blood serum is about 0.14 M NaCl. What volume of blood contains 1.0 mg of NaCl?
-
In Exercises, use a derivative routine to obtain the value of the derivative. Give the value to 5 decimal places. f'(1), where f(x) = V1 + x
-
Tabletop Exercise (15%) Develop a tabletop exercise for your organization or community. The size and scope of your exercise can be whatever you need it to be in order for you to complete the...
-
Within the framework of the Porter Five-Forces Model of Competition, describe the competitive force of rivalry among competing sellers. What are some of the factors that increase the rivalry among...
-
The XYZ Corporation has decided to make some changes to help with the work-life balance of its employees. Currently, the organization has 40 employees: 25 full-time employees and 15 part-time...
-
Grocery prices tend to play a role in how people view inflation because of how frequent these purchases are for households. In the past four years grocery prices have jumped 25% which passes overall...
-
Bogg County is a rural area whose residents rely on farming for income. The most popular crop in Bogg County is tobacco, a very labor-intensive plant. To save money, many farmers employ Hispanic...
-
In an age-discrimination case against Darmin, Inc., evidence showed that among the last 40 applicants for employment, only the 8 youngest were hired. Find the probability of randomly selecting 8 of...
-
-4 1 9. Let A = Find A-1, (A") and verify that (A")= (A-1)".
-
What is supersaturation? Under what conditions is it possible to supersaturate a solution? What is the metastable region?
-
Does the commonly reported solubility of an inorganic compound in water pertain to large crystals or small crystals? Why?
-
Can an inorganic compound have more than one form of hydrate?
-
Palisade Creek Co. is a merchandising business that uses the perpetual inventory system. The account balances for Palisade Creek Co. as of May 1, 2019 (unless otherwise indicated), are as follows:...
-
1-When accounting for an acquisition, goodwill is the difference between what two things? 2- What factors should be considered when deciding whether an acquisition should be financed with cash or...
-
What is the main friction Fluidity aims to address? REAL STATE

Study smarter with the SolutionInn App


