To what extent does the Heisenberg uncertainty principle affect your ability to specify the properties of objects
Question:
To what extent does the Heisenberg uncertainty principle affect your ability to specify the properties of objects you can see? Can you be confident about their location? Estimate the minimum uncertainty in
(a) The position of a marble of mass 1.0 g given that its speed is known to within 61.0 mm·s–1 and
(b) The speed of an electron confined to an atom with the diameter 200. pm.
ANTICIPATE You should expect the uncertainty in the position of an object as heavy as a marble to be very small but the uncertainty in the speed of an electron, which has a very small mass and is confined to a small region, to be very large.
PLAN (a) The uncertainty Δp is equal to mΔv, where Δv is the uncertainty in the speed; use Eq. 6 to estimate the minimum uncertainty in position, Δx, along the direction of the travel of the marble from ![]() (the minimum value of the product of uncertainties). (b) Assume Δx to be the diameter of the atom and use Eq. 6 to estimate Δp; use the mass of the electron inside the back cover and find Δv from Δp = mΔv.
(the minimum value of the product of uncertainties). (b) Assume Δx to be the diameter of the atom and use Eq. 6 to estimate Δp; use the mass of the electron inside the back cover and find Δv from Δp = mΔv.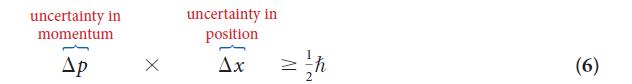
Step by Step Answer:

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman





