Use the information in Table 9C.1 to write the formula for each of the following coordination compounds:
Question:
Use the information in Table 9C.1 to write the formula for each of the following coordination compounds:
(a) Potassium hexacyanidochromate(III)
(b) Pentaamminesulfatocobalt(III) chloride
(c) Tetraamminediaquacobalt(III) bromide
(d) Sodium bisoxalato(diaqua)ferrate(III)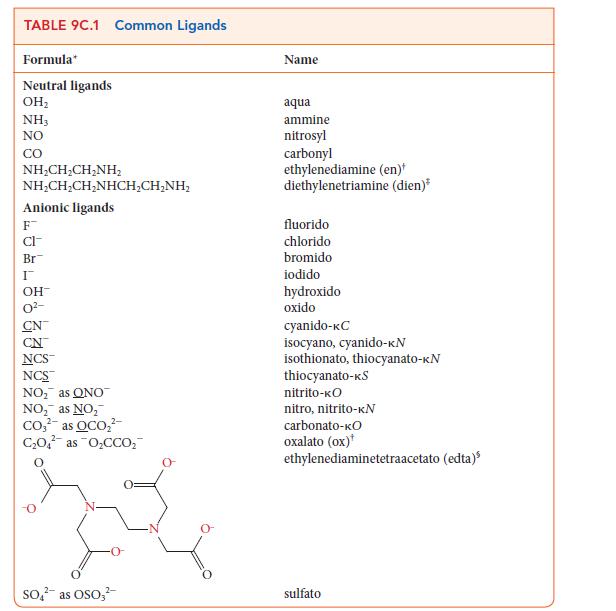
Transcribed Image Text:
TABLE 9C.1 Common Ligands Formula Neutral ligands OH₂ NH3 NO CO NHẠCH,CH_NH, NHẠCH,CH,NHCH,CHÍNH, Anionic ligands F CI- Br I OH- CN NCS™ NCS™ NO₂ as ONO NO₂ as NO₂ Co₂² as OCO₂² C₂O4 as O₂CCO₂ 2- SO² as OSO3² Name aqua ammine nitrosyl carbonyl ethylenediamine (en)* diethylenetriamine (dien)* fluorido chlorido bromido iodido hydroxido oxido cyanido-KC isocyano, cyanido-KN isothionato, thiocyanato-KN thiocyanato-KS nitrito-KO nitro, nitrito-KN carbonato-KO oxalato (ox) ethylenediaminetetraacetato (edta) sulfato
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
a KaCrCN6 b ...View the full answer

Answered By

Gabriela Rosalía Castro
I have worked with very different types of students, from little kids to bussines men and women. I have thaught at universities, schools, but mostly in private sessions for specialized purpuses. Sometimes I tutored kids that needed help with their classes at school, some others were high school or college students that needed to prepare for an exam to study abroud. Currently I'm teaching bussiness English for people in bussiness positions that want to improve their skills, and preparing and ex-student to pass a standarized test to study in the UK.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere: (a) Hexaamminechromium (III) nitrate (b) Tetraamminecarbonatocobalt (III)...
-
Write the formula for each of the following compounds, being sure to use brackets to indicate the coordination sphere: (a) Tetraaquadibromomanganese(III) perchlorate (b) Bis(bipyridyl)cadmium(II)...
-
Write the chemical formula for each of the following compounds, and indicate the oxidation state of nitrogen in each: (a) Nitric oxide (b) Hydrazine (c) Potassium cyanide (d) Sodium nitrite (e)...
-
Write a program that reads in the x - and y - coordinates in Cartesian space for the endpoints of a line segment and then determines if the line segment is parallel to the y - axis. Hint: a line is...
-
In footnote 4 we noted that the minimum-risk portfolio contained an investment of 21.4 percent in Reebok and 78.6 in Coca-Cola. Prove it.
-
Determine the forces P1 and P2 needed to hold the cable in the position shown, i.e., so segment C D remains horizontal. Also, find the maximum tension in thecable. 15 m Im 5 KN 12m
-
E ALUATING CURRENT HEALTH INSURANCE. Assess your current health insurance situation. What does your policy cover? What is excluded? Are there any gaps that you think need to be filled? Are there any...
-
Your parents are considering investing in Apple Inc. common stock. They ask you, as an accounting expert, to make an analysis of the company for them. Apples financial statements are presented in...
-
Jim Shorts Company makes clothing for schools. Sales in 20X1 were $4,740,000. Assets were as follows: Cash $ 188,000 Accounts receivable 804,000 Inventory 423,000 Net plant and equipment 585,000...
-
Predict the major products of each of the following reactions and then balance the equations: (a) FeCrO4(s) + C(s) A (b) CrO (s) + HO*(aq) (c) MnO (s) + Al(s) A
-
You are analyzing water samples from a local stream and want to use the intense red color of Fe(SCN) 2+ to measure the concentration of Fe 3+ . You need to know the formation constant (Topic 6I) for...
-
Use the eighteen rules of inference to derive the conclusions of the following symbolized arguments. 1. (S.K) DR 2. K ISOR
-
Rosita Flores owns Rosita's Mexican Restaurant in Tempe, Arizona. Rosita's is an affordable restaurant near campus and several hotels. Rosita accepts cash and checks. Checks are deposited...
-
Your second task will require you to recover a payload from the conversation. Just need 2.3. Need you to explain step by step, and concept by concept if possible. Use wireshark. Tell me your answer...
-
2. Supply for art sketchbooks at a price of $p per book can be modelled by P <10 S(p) = = textbooks. p3+p+3 p 10 (a) What is the producer revenue at the shutdown point? (b) What is the producer...
-
Patterson Company produces wafers for integrated circuits. Data for the most recent year are provided: Expected Consumption Ratios Activity Driver Wafer A Wafer B Inserting and sorting process...
-
The elementary gas-phase reaction 2A + B C+D is carried out isothermally at 450 K in a PBR with no pressure drop. The specific reaction rate was measured to be 2x10-3 L/(mol-min-kgcat) at 50C and the...
-
Use the data in TRAFFIC2.RAW for this exercise. These monthly data, on traffic accidents in California over the years 1981 to 1989, were used in Computer ExerciseC10.11. (i) Using the standard...
-
What is an insurable interest? Why is it important?
-
Why would you observe a pure rotational spectrum in the microwave region and a rotational vibrational spectrum rather than a pure vibrational spectrum in the infrared region?
-
Solids generally expand as the temperature increases. Such an expansion results from an increase in the bond length between adjacent atoms as the vibrational amplitude increases. Will a harmonic...
-
How can you observe vibrational transitions in Raman spectroscopy using visible light lasers where the photon energy is much larger than the vibrational energy spacing?
-
What is the NPV of a project that costs $34,000 today and is expected to generate annual cash inflows of $11,000 for the next 7 years, followed by a final inflow of $14,000 in year 8. Cost of capital...
-
help!!! Use the above information to calculate ending inventory using FIFO for a company that uses a perpetua/inventory system
-
Rocky Mountain Chocolate Factory (RMCF) founder and president Frank Crail employs 220 people in 361 outlets in the United States, Canada, United Arab Emirates, Japan, South Korea and Saudi Arabia. If...

Study smarter with the SolutionInn App


