Using bond energies, estimate (Delta H) for the following reaction: CH3CHOH(aq) + HOCCH3(aq) CH3CHOCCH(aq) + HO(l)
Question:
Using bond energies, estimate \(\Delta H\) for the following reaction:
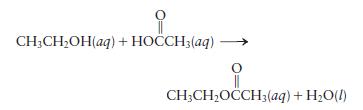
Transcribed Image Text:
CH3CHOH(aq) + HOCCH3(aq) CH3CHOCCH(aq) + HO(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
Youve provided an image of a chemical reaction and asked to estimate the change in enthalpy Delta H for the reaction using bond energies The image dep...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
When atoms of the hypothetical element X are placed together, they rapidly undergo reaction to form the X2 molecule: X(g) + X(g) X2(g) a. Would you predict that this reaction is exothermic or...
-
Estimate the value of Ho for the following reaction from bond energies (Table 9.5). H2(g) + Cl2(g) 2HCl(g). Is the reaction exothermic or endothermic? Note that the reaction involves the breaking of...
-
Create basic framework for your client using the completed ASI (I will provide the ASI document for students in class to complete). complete the following steps: Tell client's name and the...
-
Show that an emission tax and an absolute emission standard are equivalent instruments to regulate a polluting monopolist if and only if the standard is binding.
-
Let the initial position and speed of an over damped, non-driven oscillator be x0 and v0, respectively. (a) Show that the values of the amplitudes A1 and A2 in Equation 3.44 have the values A1 = 2x0...
-
Determine the optimum solution for each of the following LPs by enumerating all the basic solutions. (a) Maximize z = 2x1 - 4x2 + 5x3 - 6x4 Subject to x1 + 4x2 - 2x3 + 8x4 2 - x1 + 2x2 + 3x3 + 4x4 ...
-
1 What limitations can you see in the theories and evidence presented? For example, is Hofstedes analysis of different cultures threatened by the increasingly international outlook and interests of...
-
Use the data in GPA2.RAW for this exercise. (i) Consider the equation where colgpa is cumulative college grade point average, hsize is size of high school graduating class, in hundreds, hsperc is...
-
Lagle Corporation has provided the following information: \ table [ [ , \ table [ [ Cost per ] , [ Unit ] ] , \ table [ [ Cost per ] , [ Period ] ] ] , [ Direct materials,$ 5 . 4 0 , ] , [ Direct...
-
Give a rationale for the octet rule and the duet rule for \(\mathrm{H}\) in terms of orbitals.
-
Write Lewis structures for \(\mathrm{CO}_{3}{ }^{2-}, \mathrm{HCO}_{3}{ }^{-}\), and \(\mathrm{H}_{2} \mathrm{CO}_{3}\). When acid is added to an aqueous solution containing carbonate or bicarbonate...
-
(Matching Principle) An accountant must be familiar with the concepts involved in determining earnings of a business entity. The amount of earnings reported for a business entity is dependent on the...
-
How have you maintained your medical billing skills over the past 12 months? Include any courses or learning opportunity you used to build your current knowledge base. How did these skills help you?...
-
1. What issues does Bob Holland face as he takes over as CEO of Ben & Jerry's? Which are the most important? 2. Where is the market headed? What are the competitive influences and compare the...
-
Do you think there is a difference between diversity management and affirmative action? Provide an explanation for your response. Support your response with APA cited references. Response: Diversity...
-
1. In what ways do practical and statistical significance work together to help us understand program effects? Can one be important to aprogram evaluator withoutthe other? If so, how? If not, why...
-
How do IT metrics, measurements, productivity, and efficiency work together? Make sure you explain each word.Make sure to pick out two or three specific IT data and measures. Also, back up what you...
-
How is the utilization factor u for cogeneration plants defined? Could u be unity for a cogeneration plant that does not produce any power?
-
On October 1, 2021, Adoll Company acquired 2,600 shares of its $1 par value stock for $38 per share and held these shares in treasury. On March 1, 2023, Adoll resold all the treasury shares for $34...
-
Calculate the pH of the solution that results from mixing (a) 0.100 L of 0.050 m (CH 3 ) 2 NH(aq) with 0.280 L of 0.040 m (CH 3 ) 2 NH 2 Cl(aq); (b) 45.0 mL of 0.015 m (CH 3 ) 2 NH(aq) with 86.0 mL...
-
Morphine, C 17 H 19 O 3 N, is a potent painkiller. Suppose you are studying morphine and need to predict the pH of a morphine solution during a titration. Calculate the pH at the stoichiometric point...
-
Calculate the molar concentrations of H 2 SO 3 , HSO 3 , SO 3 2 , H 3 O + , and OH present in 0.125 m H 2 SO 3 (aq).
-
Production numbers for 2 shifts are shown. The shift supervisor of Shift 2 insists to the production manager that her operators are more productive than the ones on Shift 1. Using a confidence level...
-
In a class, the scores that students got are as shown. What are the 25, 50, 75 and 100th percentiles for the data? 84 84 98 80 89 83 85 56 85 84 84 74 84 81 83 80 45 86 67 79 81 78 76 85 83 77 86 83...
-
Number of points made by Teams A and B are shown. Which statement is true based on running the F-Test Two-Sample for Variances in the Data Analysis pack in Excel? Use a confidence level of 10% to...

Study smarter with the SolutionInn App


