Using Table 7.3, order the following bases from strongest to weakest. NO 3 - , H 2
Question:
Using Table 7.3, order the following bases from strongest to weakest.
NO3-, H2O, NH3, and C5H5N
Table 7.3
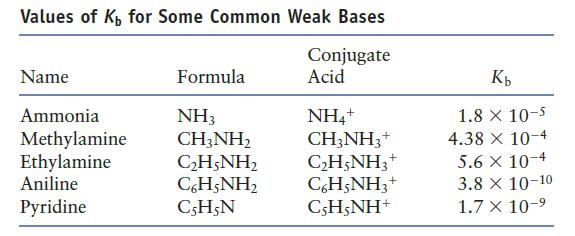
Transcribed Image Text:
Values of K₁ for Some Common Weak Bases Conjugate Acid Name Ammonia Methylamine Ethylamine Aniline Pyridine Formula NH3 CH3NH₂ CH;NH, CH;NH, C5H5N NH4+ CH3NH3 + C₂H5NH3+ C6H5NH3+ CH;NH* Kb 1.8 x 10-5 4.38 x 10-4 5.6 x 10-4 3.8 X 10-10 1.7 X 10-⁹
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
The order of the bases from strongest to weakest is as follows NO3 C5H5...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Using Table, order the following bases from strongest to weakest. NO3-, H2O, NH3, and C5H5N Table Conjugate Acid ame Formula NH3 1.8 x 10-3 4.38 x 10-4 5.6 x 104 3.8 x 10-10 1.7 10-9 NH4 Methylamine...
-
Using Table, order the following acids from strongest to weakest. HNO3, H2O, NH4+, and C5H5NH+ Table Conjugate Acid Name Formula 1.8 x 10-5 4.38 x 10-4 5.6 X 10-4 3.8 x10-10 1.7 x 109 NH3 NH4 CHNH...
-
Using bases (B:) and acids (+BH) as needed, provide a curved-arrow mechanism for the conversion of the c-amino acid serine into formaldehyde and glycine (Eq. 25.53, p. 1242). Eq. 25.53 formaldehyde...
-
Select the best response. What trend can be related to a major employer closing a business? Property values falling and people moving from a community. A rise in slips, trips and falls. New...
-
For each value of p > 1, let c(p) = Suppose that the random variable X has a discrete distribution with the following p.f.: a. For each fixed positive integer n, determine the probability that X will...
-
Many alkaloids are synthesized in nature from a precursor molecule called norlaudanosoline, which in turn appears to be derived from the condensation of amine A with aldehyde B. Formulate a mechanism...
-
Write the equations for four ratios that are used to measure how effectively a firm manages its assets. AppendixLO1
-
James Albemarle created a trust fund at the beginning of 2010. The income from this fund will go to his son Edward. When Edward reaches the age of 25, the principal of the fund will be conveyed to...
-
Calculate the cash dividends required to be paid for each of the following preferred stock issues: Required: a. The semiannual dividend on 8% cumulative preferred $70 par value, 14,000 shares...
-
You need to calculate the Average Days on Lot based on MSRP and/or Types using the Inventory data. n. Enter functions into the Average Days on Lot chart area that calculate the following: Insert a...
-
Calculate the pH of a 0.010 M solution of iodic acid (HIO 3 , Ka = 0.17).
-
What mass of HNO 3 is present in 250.0 mL of a nitric acid solution having a pH = 5.10?
-
Consider the device for the magnetic levitation of a steel ball. Obtain a design that will provide a stable response where the ball will remain within 10% of its desired position. Assume that y and...
-
Why do you think it is important to consider only relevant costs when conducting a differential analysis for a major purchase? Why not consider all possible costs in your decision? provide specific...
-
How do power dynamics and influence tactics shape decision-making processes and organizational politics within hierarchical structures ?
-
How do I answer these given the information below? Loan Amount? Loan to Value? Loan to Cost? Payment amount? Loan Balance at Maturity? Given Information: Property Cost: $1,000,000 Bank Policy on LTV:...
-
In your initial post, first do the following: Use scholarly references to define Project Management (PM), Systems Development Life Cycle (SDLC), and Application Life Cycle (AL). Then, in the same...
-
How do concepts of diversity and inclusion vary across different cultural and geographical contexts, and what strategies can multinational organizations employ to navigate these variations...
-
Does it necessarily take a minimum of 25,000 years to travel a distance of 25,000 light-years?
-
The graph of an equation is given. (a) Find the intercepts. (b) Indicate whether the graph is symmetric with respect to the x-axis, the y-axis, or the origin. -3 6 -6 3 x
-
Suppose the reaction system UO2(s) + 4HF(g) UF4(g) + 2H2O(g) has already reached equilibrium. Predict the effect that each of the following changes will have on the equilibrium position. Tell...
-
Consider the reaction: Fe3+(aq) + SCN2(aq) FeSCN2+(aq) How will the equilibrium position shift if a. Water is added, doubling the volume? b. AgNO3(aq) is added? (AgSCN is insoluble.) c. NaOH(aq) is...
-
Chromium(VI) forms two different oxyanions, the orange dichromate ion (Cr2O7-2), and the yellow chromate ion (CrO4-2). (See the photos below.) The equilibrium reaction between the two ions is...
-
Production costs that are not attached to units that are sold are reported as: Cost of goods sold Selling expenses Administrative costs Inventory
-
Please show workings :) Oxford Company has limited funds available for investment and must ration the funds among four competing projects. Selected information on the four projects follows: Life of...
-
ASDA Company reported the following activity in the Assembly Department for the month of May : Units Percent Completed Materials Conversion Work in process, May 1 360 50 % 10 % Units started into...

Study smarter with the SolutionInn App


