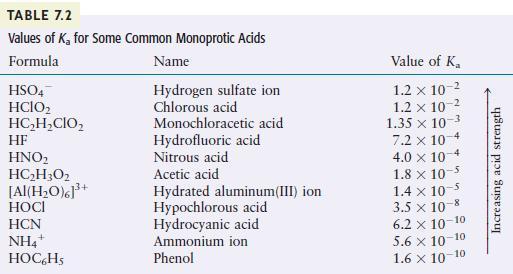
Using the K a K a values given in Table 7.2, calculate the concentrations of all species
Question:
Using the Ka values given in Table 7.2, calculate the concentrations of all species present and the pH for each of the following.
a. 0.20MHOCl
b. 1.5MHOC6H5
c. 0.020MHF
Transcribed Image Text:
TABLE 7.2 Values of K for Some Common Monoprotic Acids Formula Name Hydrogen sulfate ion Chlorous acid HSO4 HCIO HCHCIO HF HNO HCH3O2 [Al(HO)]+ HOCI HCN NH4+ HOC6H5 Monochloracetic acid Hydrofluoric acid Nitrous acid Acetic acid Hydrated aluminum(III) ion Hypochlorous acid Hydrocyanic acid Ammonium ion Phenol Value of K 1.2 x 10- 1.2 x 10-2 1.35 x 10-3 7.2 x 10-4 4.0 x 10 1.8 x 10-5 1.4 x 10- 3.5 x 10-8 6.2 10-10 5.6 10-10 1.6 10-10 Increasing acid strength
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
The question involves calculating the concentrations of all species present and the pH for different solutions of monoprotic acids using given Ka valu...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Calculate the concentrations of all species in a 0.100 M H3PO4 solution.
-
The gamma distribution may be written in several different (but mathematically equivalent) forms. Excel uses the following form for the two-parameter gamma distribution in its functions GAMMADIST...
-
Write a short paper describing your current (or most recent) job and the compensation system in place at your current (or most recent) place of employment. Dont use the name of a company, just...
-
West State Furniture (WSF) manufactures desks and desk chairs using two departments within a single facility. The West Department produces the desks, and the State Department produces the chairs. WSF...
-
Determine the minimum multiplication of a telescope with diameter of objective D = 5.0 cm with which the resolving power of the objective is totally employed if the diameter of the eye's pupil is do...
-
Consider a small country applying a tariff t such as in Figure 8-5. Instead of a tariff on all units imported, however, we will suppose that the tariff applies only to imports in excess of some quota...
-
List steps HR can take to support virtual work.(pp. 382385)
-
a. Find the present values of the following cash flow streams. The appropriate interest rate is 8%. b. What is the value of each cash flow stream at a 0% interestrate? Cash Stream A Cash Stream B...
-
Adlusted balance: $4,830 PR 7-4A Bank reconciliation and entries Ob5 The cash account for Coastal Bike Co, at October 1, 2019, indicated a balance of 55.140. During October, the total cash deposited...
-
Monochloroacetic acid \(\left(\mathrm{HC}_{2} \mathrm{H}_{2} \mathrm{ClO}_{2}ight)\) is a skin irritant that is used in "chemical peels" intended to remove the top layer of dead skin from the face...
-
List the major species present in \(0.250 \mathrm{M}\) solutions of each of the following acids, and then calculate the \(\mathrm{pH}\) for each. a. \(\mathrm{HBr}\) b. \(\mathrm{HClO}_{4}\) c....
-
In the last chapter you added and modified some forms for Kelly's Boutique. She would now like you to create some new reports. Make the following changes for Kelly using the ch 12-03_student_name...
-
Idenfity whether the following book - tax adjustments are permanent or temporary differences. ( a ) Federal Income Tax Expense ( b ) Depreciation Expense ( c ) Accrued Compensation ( d ) Dividends...
-
2 . ) Pozycki, LLC has reported losses of $ 1 0 0 , 0 0 0 per year since its founding in 2 0 1 6 . For 2 0 2 3 , Pozycki anticipates a profit of about $ 1 0 0 , 0 0 0 . There are 3 equal members of...
-
Elena is a single taxpayer for tax year 2023. On April 1st, 2022, Elena's husband Nathan died. On July 13, 2023, Elena sold the residence that Elena and Nathan had each owed and used as their...
-
Rodriguez Corporation issues 12,000 shares of its common stock for $56,600 cash on February 20. Prepare journal entries to record this event under each of the following separate situations. 1. The...
-
Problem 3: A large rectangular plate is loaded in such a way as to generate the unperturbed (i.e. far-field) stress field xx = Cy; yy = -C x; Oxy = 0 The plate contains a small traction-free circular...
-
(a) Unregulated, how much will each firm pollute? Why? What will total pollution be? What will each firm's profits be? (b) The Department of Environmental Quality (DEQ) would like to reduce pollution...
-
If the annual fixed costs are 54,000 dinars, the occupation expense represents 20%, the contribution margin is 25%, and the unit selling price is 40 dinars. Required: Calculate the closing point of...
-
The uncertainty principle has negligible consequences for macroscopic objects. However, the properties of nanoparticles, which have dimensions ranging from a few to several hundred nanometers, may be...
-
The energy levels of a particle of mass m in a two-dimensional square box of side L are given by (n 1 2 + n 2 2 ) h 2 /8 mL 2 . Do any of these levels have the same energy? If so, find the values of...
-
Which of the following happens when the frequency of electromagnetic radiation decreases? Explain your reasoning. (a) The speed of the radiation decreases. (b) The wavelength of the radiation...
-
Choose two stocks from the same industry to minimize the influence of other confounding factors. You choose the industry that you are relatively more familiar with, and then estimate the implied...
-
why should Undertake research to review reasons for previous profit or loss?
-
A pension fund's liabilities has a PV01 of $200 million. The plan has $100 billion of assets with a weighted average modified duration of 8. The highest duration bond that the plan can invest in has...

Study smarter with the SolutionInn App


