Question: Using the plots in Fig. 16.48(b), determine whether water or diethyl ether has the smaller enthalpy of vaporization. Figure 16.48(b) In (Pvap) (b) Water 1/T
Using the plots in Fig. 16.48(b), determine whether water or diethyl ether has the smaller enthalpy of vaporization.
Figure 16.48(b)
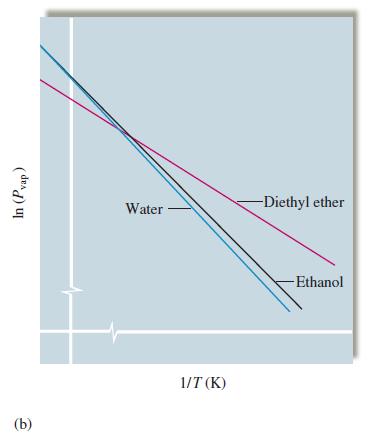
In (Pvap) (b) Water 1/T (K) -Diethyl ether -Ethanol
Step by Step Solution
3.34 Rating (163 Votes )
There are 3 Steps involved in it
When In Pvap is plotted versus 1T the slope of the resulting straight line is AHv... View full answer

Get step-by-step solutions from verified subject matter experts


