When lead(II) sulfide is treated with hydrogen peroxide, the possible products are either lead(II) sulfate or lead(IV)
Question:
When lead(II) sulfide is treated with hydrogen peroxide, the possible products are either lead(II) sulfate or lead(IV) oxide and sulfur dioxide.
(a) Write balanced equations for the two reactions.
(b) Use data from Appendix 2A to decide which possibility is more likely.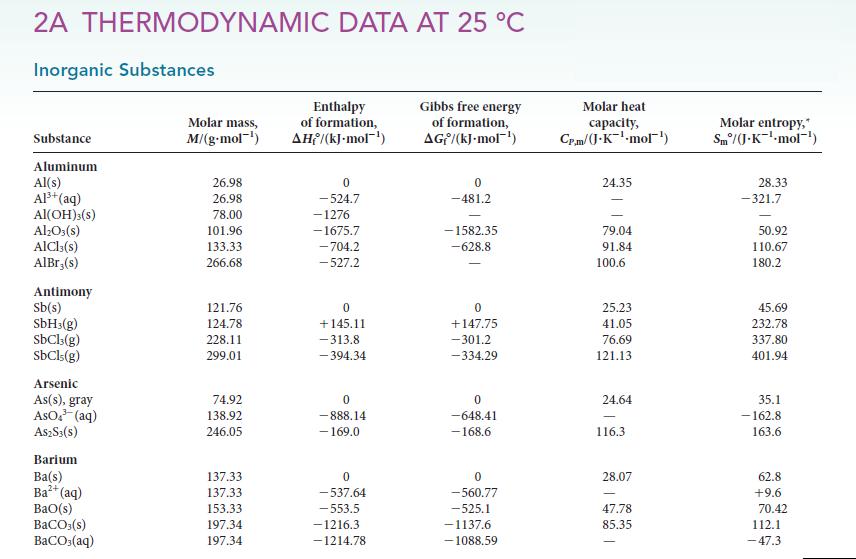
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(s) Al₂O3(s) AICI,(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls(g) Arsenic As(s), gray AsO4³ (aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ.mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ-mol-¹) 0 -481.2 -1582.35 -628.8 0 +147,75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J-K¹-mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy, Sm/(J.K¹-mol-¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The two possible balanced equations for the reactions are as follows Equation 1 PbS 4HO PbSO 4HO Q ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write balanced equations for each of the following reactions (some of these are analogous to reactions shown in the chapter). (a) Aluminum metal reacts with acids to form hydrogen gas. (b) Steam...
-
Write balanced equations for the following reactions: (a) Barium oxide with water, (b) Iron (II) oxide with perchloric acid, (c) Sulfur trioxide with water, (d) Carbon dioxide with aqueous sodium...
-
Write balanced equations for each of the following reactions. (a) When mercury(II) oxide is heated, it decomposes to form O2 and mercury metal. (b) When copper(II) nitrate is heated strongly, it...
-
Suppose you observe the following exchange rates: 1 = $1.50; 120 = $1.00. Calculate the euro-yuan exchange rate. Group of answer choices a. 80 = 1.00 b. 1 = 2.50 c. 133.33 = 1.00 d. 1.00 = 180
-
Draw a figure like Figure 2.1 to represent the following situation. a. A firm starts out with $10 million in cash. b. The rate of interest r is 10 percent. c. To maximize NPV the firm invests today...
-
Walk across the room in such a way that, after getting started, your velocity is negative, but your acceleration is positive. (a) Describe how you did it. (b) Sketch a graph of v versus t for your...
-
Table 16.2 (after Problem 7) lists the work W associated with several human activities. For walking, the table lists W/t = 50 W, where t is the time spent walking. Use Newtons laws of motion to...
-
China is a major producer of grains, such as wheat, corn, and rice. In 2008 the Chinese government, concerned that grain exports were driving up food prices for domestic consumers, imposed a tax on...
-
Question 62 (10 points) All changes in equity during a period, except those from owners' investments and dividends, are considered part of a) Comprehensive Income Ob) Comprehensive Securities c)...
-
The interhalogen IFx can be made only by indirect routes. For example, xenon difluoride gas can react with iodine gas to produce IF x and xenon gas. In one experiment, xenon difluoride is introduced...
-
The interhalogen ClF x has been used as a rocket fuel. It reacts with hydrazine to form the gases hydrogen fluoride, nitrogen,and chlorine. In one study of this reaction, ClF x gas is introduced into...
-
On February 1, Jacher Company, a U.S. company, purchased inventory on credit from a British Company for 50,000. Jacher properly recorded its inventory and accounts payable at $90,000, based on an...
-
Contract for construction crew and equipment 8 Build parking lots Exterior lighting 11 7 20 12 Build foundation Start Interior Interior 12 9 electrical Final wiring finish Purchase 8 14 12 material...
-
Mad Hatter Enterprises purchased new equipment for $369,000, terms f.o.b. shipping point. Other costs connected with the purchase were as follows: State sales tax Freight costs Insurance while in...
-
Write down a C program that takes runs scored by a batsman and prints the status according to the following policy: Runs scored >80 50-79 30-49 10-29 <10 Grade Excellent 4 Very Good Good Average Poor
-
Consider the standard two-period maximization problem for investor j over s states of nature: Subject to S max u(c) + (s)u(c;}(s)) S=1 Cjo + q(s) C; (s) = Wjo +244) S=1 where all terms are as defined...
-
At what point should a leader cease gathering data, take the risk, and simply make the decision? Support your position.
-
Use CONSUMP.RAW for this exercise. One version of the permanent income hypothesis (PIH) of consumption is that the growth in consumption is unpredictable. [Another version is that the change in...
-
Consider the circuit of Fig. 7.97. Find v0 (t) if i(0) = 2 A and v(t) = 0. 1 3 ett)
-
Methane belongs to the T d group. The reducible representation for the vibrational modes is reducible = A 1 + E + 2T 2 . a. Show that the A 1 and T 2 representations are orthogonal to each other and...
-
Use the 3 Ã 3 matrices for the C 2v group in Equation (27.2) to verify the associative property for the following successive operations: a. b. ,(6,C2) = (6,6-)C () = 6,(2) %3D
-
Use the logic diagram of Figure 27.2 to determine the point group for allene. Indicate your decision-making process as was done in the text for NH3 1. linear? 2. C n axis? 3. more than 1C n axis? 4....
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...
-
On Dec 31 2020, Bernice Melson, a partner in ABC Communications, had an ending capital balance of $49,000. Her share of the partnership's profit was $18,000; she made investments of $12,000 and had...
-
Q2R. on account for each depreciable asset. During 2024, Jane VIIS nsactions.) i More Info Apr. 1 Purchased office equipment. 5111,000. Paid 581,000 cash and financed the remainder Jan. 1 with a note...

Study smarter with the SolutionInn App


