Which of the following indicators in Table 6H.2 could you use for a titration of 0.20 m
Question:
Which of the following indicators in Table 6H.2 could you use for a titration of 0.20 m NH3(aq) with 0.20 m HCl(aq):
(a) Bromocresol green;
(b) Methyl red;
(c) Phenol red;
(d) Thymol blue? Explain your selections.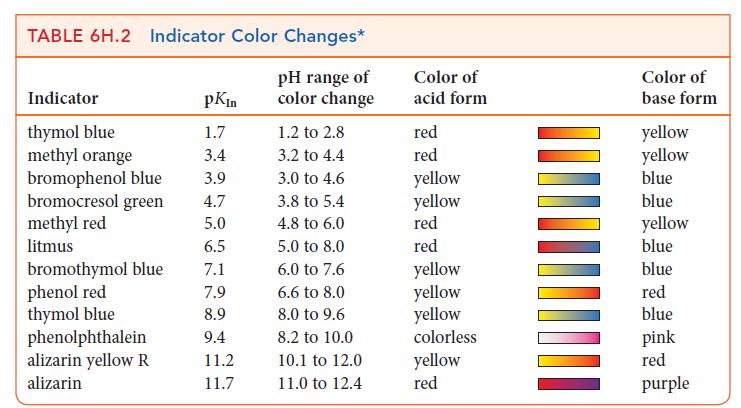
Transcribed Image Text:
TABLE 6H.2 Indicator Color Changes* pH range of color change Indicator thymol blue methyl orange bromophenol blue bromocresol green methyl red litmus bromothymol blue phenol red thymol blue phenolphthalein alizarin yellow R alizarin pKin 1.7 3.4 3.9 4.7 5.0 6.5 7.1 7.9 8.9 9.4 11.2 11.7 1.2 to 2.8 3.2 to 4.4 3.0 to 4.6 3.8 to 5.4 4.8 to 6.0 5.0 to 8.0 6.0 to 7.6 6.6 to 8.0 8.0 to 9.6 8.2 to 10.0 10.1 to 12.0 11.0 to 12.4 Color of acid form red red yellow yellow red red yellow yellow yellow colorless yellow red Color of base form yellow yellow blue blue yellow blue blue red blue pink red purple
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Given the pH at the equivalence point is approximately 237 thymol blue would be the most suitable ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
You would like to invest in one of the three available investment plans: money market, bonds, or stocks. The payoffs (profits) of each plan under two possible future economic conditions, PE (poor...
-
A series of titrations of lactic acid, CH 3 CH(OH)COOH (pK a = 3.86) is planned. About 1.00 mmol of the acid will be titrated with NaOH(aq) to a final volume of about 100 mL at the equivalence point....
-
Select indicators from Table 10-3 that would be useful for the titrations in Figures 10-1 and 10-2 and the pK a = 8 curve in Figure 10-3. Select a different indicator for each titration and state...
-
(a) A circular diaphragm 60 cm in diameter oscillates at a frequency of 25 kHz as an underwater source of sound used for submarine detection. Far from the source, the sound intensity is distributed...
-
Find the mass of a lead sphere of radius 2 cm.
-
The rate constant for the decomposition of a certain substance is 1.70 x 10-2 dm3 mol-1 S-1 at 24C and 2.01 X 10-2 dm3 mol-1 S-1 at 37"C. Evaluate the Arrhenius parameters of the reaction.
-
2. Evaluate the following limits when they exist, (a) (b) (c) (d) (e) I, x + 1 1m 2 ' x-+O+ X - 2x I, X3 - 3x + 2 1m 3 x-+l- X - 1 lim (x2 + 1) sinx, X-+7f+ I, x 1m -, x-+O+ Ixl tanx lim x-+7f/2- X
-
Sherrys Meats, a regional meat wholesaler and retailer, needs to collect up-to-date information on how much of each meat product it has in each store. It will then use that information to schedule...
-
Crane Companv reportad tha followinc pretax financlal income foal for the vears 20102022. (a) and enter Ofor the omounta)
-
What specifically, is direct response advertising? What makes it unique from all other types advertising?
-
(a) What is the approximate chemical formula of rust? (b) What is the oxidizing agent in the formation of rust? (c) How does the presence of salt accelerate the rusting process?
-
(a) What must be the ratio of the molar concentrations of CO 3 2 and HCO 3 ions in a buffer solution having a pH of 11.0? (b) What mass of K 2 CO 3 must be added to 1.00 L of 0.100 m KHCO 3 (aq) to...
-
Refer to Fig. 10. Describe what happens to cos t as t increases from 0 to . (a) P P (b) P P (d) Figure 10 Movement along the unit circle. (e) P (c)
-
Solve (c) 8 WI n=1 5 cos n N5
-
- Pierce Company reported net income of $160,000 with income tax expense of $19,000 for 2020. Depreciation recorded on buildings and equipment amounted to $80,000 for the year. Balances of the...
-
ABC Company had the following results as of 12/31/2020: ABC's hurdle rate is 10% CONTROLLABLE REVENUE CONTROLLABLE COST CONTROLLABLE ASSETS CONTROLLABLE INCOME 21. What is the division's margin? A....
-
A gray kangaroo can bound across a flat stretch of ground with each jump carrying it 10 m from the takeoff point. If the kangaroo leaves the ground at a 20 angle, what are its (a) takeoff speed and...
-
Since 1900, many new theories in physics have changed the way that physicists view the world. Create a presentation that will explain to middle school students why Quantum Mechanics is important, how...
-
For the Hamming code shown in Figure 5.10, show what happens when a check bit rather than a data bit is in error? Bit position Position number Data bit Check bit 12 10987 6 53 2 1100 101 1010 1001...
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
A balloon filled with 11.50 L of Ar at 18.7C and one atm rises to a height in the atmosphere where the pressure is 207 Torr and the temperature is 32.4C. What is the final volume of the balloon?...
-
Carbon monoxide competes with oxygen for binding sites on the transport protein hemoglobin. CO can be poisonous if inhaled in large quantities. A safe level of CO in air is 50. parts per million...
-
The total pressure of a mixture of oxygen and hydrogen is 1.65 atm. The mixture is ignited and the water is removed. The remaining gas is pure hydrogen and exerts a pressure of 0.190 atm when...
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


