Which of the following ligands do you expect to form chelating complexes? Explain your reasoning. (a) Bipyridine
Question:
Which of the following ligands do you expect to form chelating complexes? Explain your reasoning.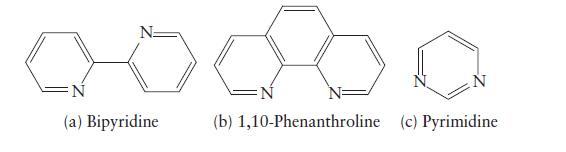
Transcribed Image Text:
(a) Bipyridine (b) 1,10-Phenanthroline (c) Pyrimidine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
Of the three ligands in the imageI expect that 110phenanthroline b is the most likely to form chelat...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Hemoglobin contains one heme group per subunit. The heme group is an Fe 2+ complex that is coordinated to the four N atoms in a porphyrin ligand in a square planar arrangement and to an N atom on the...
-
Which of the following ligands are capable of linkage isomerism? Explain your answrer. SCN, N3, NO2, NH2CH CH2NH2, OCN-,I
-
Discuss the current tax treatment of capital gains under the personal income tax. Why do some economists argue that reduction in the rate of taxation of capital gains can actually increase tax...
-
Q6) From the product_t table, return the number of rows of products with a natural ash finish OR a cherry finish Q7) From the customer_t table, return all entries with a postal code beginning with 9,...
-
Suppose a process is defined to behave as described in Figure. Design and implement a resource manager for reusable resources. When multiple processes are blocked on a resource and one or more units...
-
Percival Hygiene has $10 million invested in long-term corporate bonds. This bond portfolios expected annual rate of return is 9 percent, and the annual standard deviation is 10 percent. Amanda...
-
A more general analysis of refracting surfaces would include the case of refraction of light at a spherical interface such as a fishbowl. Similar to the thin lens equation, the expression relating...
-
The condensed product-line income statement for Suffolk China Ware Company for the month of December is as follows: Fixed costs are 15% of the cost of goods sold and 40% of the selling and...
-
The following data were taken from the records of Menendez Company: Current assets $ 5,000 Property, plant, and equipment 10,000 Current liabilities 3,500 Long-term liabilities 5,000 Stockholders'...
-
(a) What reducing agent is used in the production of iron from its ore? (b) Write chemical equations for the production of iron in a blast furnace. (c) What is the major impurity in the product of...
-
(a) Which types of ligands are -acid ligands in general, strong field or weak field? (b) Which types of ligands are -base ligands in general, strong field or weak field?
-
Discuss methods by which long- and short-term absence can be minimised
-
Machine-hours required to support estimated production Fixed manufacturing overhead cost Variable manufacturing overhead cost per machine-hour Required: 1. Compute the plantwide predetermined...
-
Assume now that a new firm (firm N) discovers and patents a more efficient technology, summarized by thetotal cost function C = 10q. The new technology can be used only by the new firm, which enters...
-
1. How has Dell used virtual integration to become an industry leader? Dell has used virtual integration to become an industry leader by leveraging its global suppliers to reduce costs and provide...
-
3) Consider the asset pricing model with uncertainty in the slide. We derived the asset prices as Pb = Ps = - [nu' (y+Yn + e) + (1 )u' (y + y + e)] u'(e1) [nu' (y +n + ) + (1 )u' (y + y + e2)] u'(e1)...
-
Amazon is considered a leader in managing its supply chain. Describe in detail two parts of Amazon's Supply Chain Management that you see as critical to their success. Please provide your reasoning...
-
In Problem 4.11, the R-squared from estimating the model log(salary) = (0 + (1logi (sales) + (2 log(mktval) + (3 profmarg + (4 ceoten + (5 fomten + u, Using the data in CEOSAL2.RAW, was R2 = .353 (n...
-
Danielle has an insurance policy with a premium of $75 per month. In September she is in an accident and receives a bill worth $2990 for the repair of her own property. Her deductible is $250 and her...
-
Consider para-H 2 (B = 60.853 cm -1 ) for which only even J levels are available. Evaluate the rotational partition function for this species at 50. K. Perform this same calculation for HD (B =...
-
Calculate the rotational partition function for the interhalogen compound F 35 Cl (B = 0.516 cm 1 ) at 298 K.
-
Calculate the rotational partition function for 35 Cl 2 (B 0.244 cm -1 ) at 298 K.
-
X Your answer is incorrect. Flounder Consulting Corp. company records revealed the following for the current year: What was the net cash flow from operating activities for the year? $ 0 $ 9 8 0 0...
-
Assume that interest rate parity holds. The U.S. fiveyear interest rate is 0.08 annualized, and the Mexican fiveyear interest rate is 0.05 annualized. Todays spot rate of the Mexican peso is $0.21....
-
find the NSP of a whole life insurance.6 with $100,000 Death benefits, for a female aged 105 years, if i=10%? (use Australian life Tables 2005-07) find the NSP of a whole life insurance.6 with...

Study smarter with the SolutionInn App


