Write a balanced chemical equation for the formation reaction of (a) HCl(g); (b) C 6 H 6
Question:
Write a balanced chemical equation for the formation reaction of
(a) HCl(g);
(b) C6H6(l);
(c) CuSO4 · 5H2O(s);
(d) CaCO3(s, calcite). For each reaction, determine ΔH°, ΔS°, and ΔG° from data in Appendix 2A.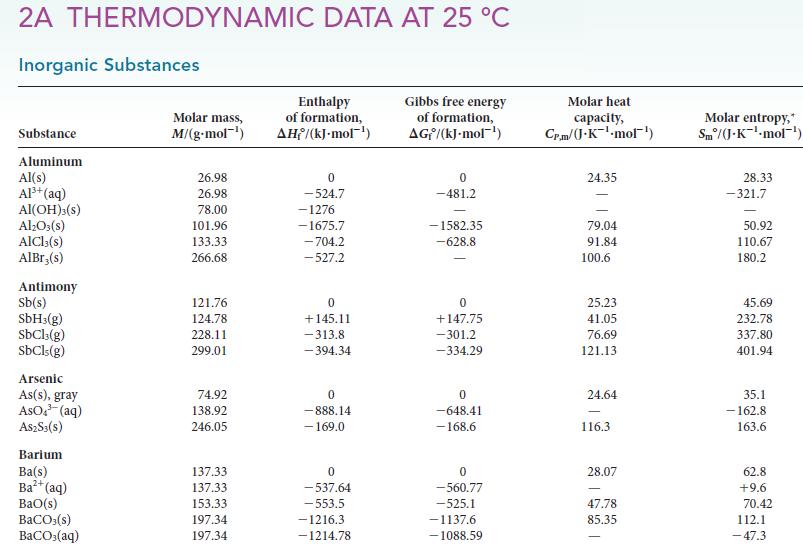
Transcribed Image Text:
2A THERMODYNAMIC DATA AT 25 °C Inorganic Substances Substance Aluminum Al(s) Al³+ (aq) Al(OH)3(S) Al₂O3(s) AlCl3(s) AlBr,(s) Antimony Sb(s) SbH3(g) SbCl3(g) SbCls (g) Arsenic As(s), gray AsO³(aq) A$2S3(S) Barium Ba(s) Ba²+ (aq) BaO(s) BaCO3(s) BaCO3(aq) Molar mass, M/(g.mol-¹) 26.98 26.98 78.00 101.96 133.33 266.68 121.76 124.78 228.11 299.01 74.92 138.92 246.05 137.33 137.33 153.33 197.34 197.34 Enthalpy of formation, AH/(kJ-mol-¹) 0 -524.7 -1276 -1675.7 -704.2 -527.2 0 +145.11 -313.8 -394.34 0 -888.14 - 169.0 0 -537.64 -553.5 -1216.3 -1214.78 Gibbs free energy of formation, AG/(kJ.mol-¹) 0 -481.2 -1582.35 -628.8 0 +147.75 -301.2 -334.29 0 -648.41 -168.6 0 -560.77 -525.1 -1137.6 -1088.59 Molar heat capacity, Cr.m/(J.K¹.mol¹) 24.35 79.04 91.84 100.6 25.23 41.05 76.69 121.13 24.64 116.3 28.07 47.78 85.35 Molar entropy,* Sm/(J-K-¹-mol¹) 28.33 -321.7 50.92 110.67 180.2 45.69 232.78 337.80 401.94 35.1 -162.8 163.6 62.8 +9.6 70.42 112.1 -47.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Lets write the balanced chemical equations for the formation reactions of the compounds youve mentioned and determine their standard enthalpy H entrop...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Principles The Quest For Insight
ISBN: 9781464183959
7th Edition
Authors: Peter Atkins, Loretta Jones, Leroy Laverman
Question Posted:
Students also viewed these Sciences questions
-
Write a balanced chemical equation for the formation reaction of (a) NH 3 (g); (b) H 2 O(g); (c) CO(g); (d) NO 2 (g). For each reaction, determine H, S, and G from data in Appendix 2A. 2A...
-
(a) Write a balanced chemical equation for the formation of 1 mol of MgO(s) from the elements in their standard states. (Find the value for f H for MgO(s) in Appendix L.) (b) What is the standard...
-
Write a balanced chemical equation for each neutralization reaction in Exercise 3. In exercise 5 a. HCl and KOH b. H2SO4 and KOH c. H3PO4 and Ni(OH)2
-
Wilburton Hospital is investigating the possibility of investing in new dialysis equipment. Two local manufacturers of this equipment are being considered as sources of the equipment. After-tax cash...
-
The cost of merchandise sold for Kohl's Corporation for a recent year was $9,891 million. The balance sheet showed the following current account balances (in millions): Determine the amount of cash...
-
Refer to the Journal of Food Engineering (Sep. 2013) study of the characteristics of fried sweet potato chips, Exercise 7.78. Recall that a sample of 6 sweet potato slices fried at 130 using a vacuum...
-
2-2. What are examples of a functional level in an organization?
-
Journalize the following sales transactions for Antique Mall. Explanations are not required. The company estimates sales returns at the end of each month. Jan. 4 Sold $16,000 of antiques on account,...
-
What is the balance of supplies expense after the closing entries are made? Account Title Cash Accounts Receivable Office Supplies Prepaid Rent Prepaid Insurance Accounts Payable Capital, C. Moore...
-
Suppose that 320.0 g of ethanol at 18.0C is mixed with 120.0 g of ethanol at 56.0 C at constant atmospheric pressure in a thermally insulated vessel. Calculate S and S tot for the process.
-
(a) Calculate the work associated with the isothermal, reversible expansion of 1.000 mol of ideal gas molecules from 7.00 L to 15.50 L at 25.0 C. (b)Calculate the work associated with the...
-
What effect will the auditors preliminary assessment of control risk have on the extent of tests of control?
-
How would you explain the following code in plain English? boxplot(age ~ gender, data = donors) Question 8 options: Make a boxplot comparing gender grouped by age, using the donors dataset Make two...
-
Vision Consulting Inc. began operations on January 1, 2019. Its adjusted trial balance at December 31, 2020 and 2021 is shown below. Other information regarding Vision Consulting Inc. and its...
-
A Jeans maker is designing a new line of jeans called Slams. Slams will sell for $290 per unit and cost $182.70 per unit In variable costs to make. Fixed costs total $68,500. (Round your answers to 2...
-
NAME: Week Two Define Claim in your own words Explain the difference between a discussion and an argument. Summarize the characteristics of a claim (Listing is not summarizing) Define Status Quo in...
-
1.How do you think major stores such as Walmart will change in the future under this new retail renaissance? 2.What are some changes that you would suggest in traditional retail stores to attract...
-
Calculate the nuclear binding energy (in J) and the binding energy per nucleon of the following isotopes: (a) 73Li (7.01600 amu) and (b) 3517Cl(34.95952 amu).
-
Describe basic managerial approaches to implementing controls and how these are implemented.
-
At a certain temperature, the vapor pressure of pure benzene (C 6 H 6 ) is 0.930 atm. A solution was prepared by dissolving 10.0 g of a nondissociating, nonvolatile solute in 78.11 g of benzene at...
-
The vapor pressure of a solution containing 53.6 g glycerin (C 3 H 8 O 3 ) in 133.7 g ethanol (C 2 H 5 OH) is 113 torr at 40C. Calculate the vapor pressure of pure ethanol at 40C assuming that...
-
In terms of Raoults law, distinguish between an ideal liquidliquid solution and a nonideal liquidliquid solution. If a solution is ideal, what is true about H soln , T for the solution formation, and...
-
Saly paid $52,000 a year paid on a weekly basis. last pay she had $250 withheld in Income Tax, $48.97 for CPP and $15.80 for EI. an additional $and 25.00 in tax are deducted each pay. She allowed to...
-
Required information [The following information applies to the questions displayed below.] Dain's Diamond Bit Drilling purchased the following assets this year. Asset Drill bits (5-year) Drill bits...
-
Which of the following partnership items are not included in the self-employment income calculation? Ordinary income. Section 179 expense. Guaranteed payments. Gain on the sale of partnership...

Study smarter with the SolutionInn App


