Conduct a safety review for the design of the system described in Example 11-2. This reactor is
Question:
Conduct a safety review for the design of the system described in Example 11-2. This reactor is used to polymerize ethylene oxide to form polyols.
Example 11-2
Transcribed Image Text:
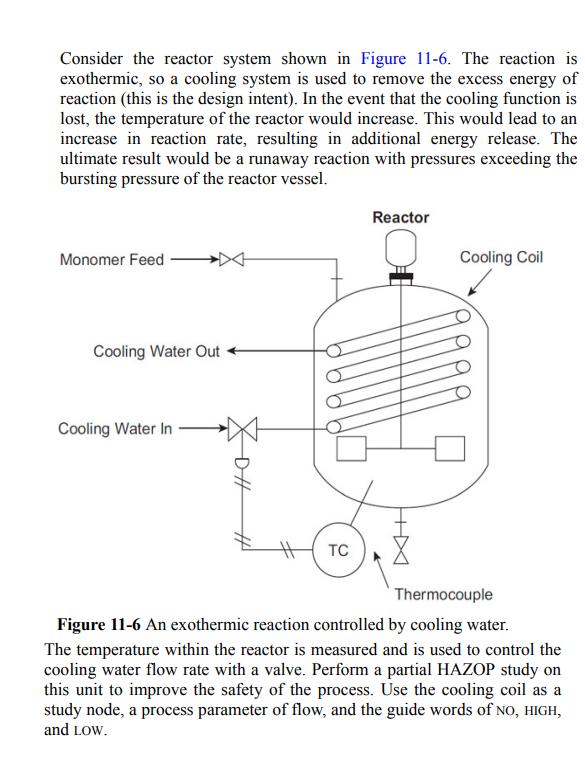
Consider the reactor system shown in Figure 11-6. The reaction is exothermic, so a cooling system is used to remove the excess energy of reaction (this is the design intent). In the event that the cooling function is lost, the temperature of the reactor would increase. This would lead to an increase in reaction rate, resulting in additional energy release. The ultimate result would be a runaway reaction with pressures exceeding the bursting pressure of the reactor vessel. Monomer Feed Cooling Water Out Cooling Water In TC Reactor X Cooling Coil Thermocouple Figure 11-6 An exothermic reaction controlled by cooling water. The temperature within the reactor is measured and is used to control the cooling water flow rate with a valve. Perform a partial HAZOP study on this unit to improve the safety of the process. Use the cooling coil as a study node, a process parameter of flow, and the guide words of NO, HIGH, and LOW.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
NO Cooling Water Flow Hazard If there is a complete failure in the cooling water flow NO flow the reactor temperature will continue to rise unchecked ...View the full answer

Answered By

Geoffrey Isaboke
I am an industrious tutor with a 5-yr experience in professional academic writing. I have passion for History and Music and I have good knowledge in Economics
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
A good management practice is to set objectives before safety reviews are conducted. The objectives should include the timing for completing the objectives. Develop objectives for a safety review for...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
Income statements for the current year and one year ago follow. Assume that all sales are on credit. For Year Ended December 31 Sales Cost of goods sold Other operating expenses Interest expense...
-
A thin steel wire of uniform cross section is bent into the shape shown, where arc BC is a quarter circle of radius R. Locate its center of gravity. 300 mm I80 am 100 m
-
What is organizational commitment? What three components have emerged to help better explain the complexities of commitment? Why may an understanding of organizational commitment be especially...
-
Gross domestic product. Refer to The Economy 2020 report estimating gross domestic product (GDP) in 2018 for 12 different industries in Newfoundland and Labrador, Exercise 2.30 (p. 70). Suppose the...
-
Internal Control Questionnaire Items: Assertions, Tests of Controls, and Possible Errors or Frauds. Following is a selection of items from the payroll processing internal control questionnaire in...
-
Assume the following statement appears in the media: Tech giants such as Google and Facebook have been criticised for not paying income tax in New Zealand proportional to their local advertising...
-
An operator needs to charge 5 kilograms of a catalyst into a batch reactor (Reactor A) 3 hours after the start of the batch. List 10 or more ways the operator can fail to perform this task correctly,...
-
The "fail safe" concept is used to specify the position (fail closed or fail open) of all process valves in the event of a utility failure. The specified fail open or fail closed puts the process in...
-
The density of a chemical solution is normally distributed with mean 0.0046 and variance 9.6 10-8. (a) What is the probability that the density is less than 0.005? (b) What is the probability that...
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
Charlene wrote a letter to Rachel offering to sell her car, a Proton Saga, for RM 60,000. The letter reached Rachel on 25. 11.2020. Rachel sent her letter of acceptance at 3 p.m. on the same day....
-
Data for the risk premium sensitivities (b, s, and h) as well as the beta coefficient for the CAPM of two companies are listed in the following table: Company b s h ERP SMBP HMLP Beta Alpha 1.1114...
-
Free-Response Questions 1. m Initial position eviribrA ARAL m Incline raised to 0 <0max pr A block of mass m is initially at rest on a rough board, which is initially horizontal on a tabletop. The...
-
A picture frame sits atop a bookshelf. When the bookshelf is bumped, the frame tumbles to the floor, landing after 0.64 s. How tall is the bookshelf?
-
A data set consists of 20 values that are fairly close together. Another value is included, but this new value is an outlier (very far away from the other values). How is the standard deviation...
-
1. What are some current issues facing Saudi Arabia? What is the climate for doing business in Saudi Arabia today? 2. Is it legal for Auger's firm to make a payment of $100,000 to help ensure this...
-
Why does the solubility of a salt of a basic anion increase with decreasing pH? Write chemical reactions for the minerals galena (PbS) and cerussite (PbCO 3 ) to explain how acid rain mobilizes...
-
Considering just acid-base chemistry, not ion pairing and not activity coefficients, find the pH and concentrations of species in 1.00 L of solution containing 0.020 mol arginine, 0.030 mol glutamic...
-
A solution containing 0.139 mmol of the triprotic acid tris(2-aminoethyl)amine 3HCl plus 0.115 mmol HCl in 40 mL of 0.10 M KCl was titrated with 0.490 5 M NaOH to measure acid dissociation...
-
Q1) The equity of Washington Ltd at 1 July 2020 consisted of: Share capital 500 000 A ordinary shares fully paid $1 500 000 400 000 B ordinary shares issued for $2 and paid to $1.50 600 000 General...
-
out The following information relates to Questions 1 to 2. The management accountant of a furniture manufacturer is developing a standard for the labour cost of one massage chair. When operating at...
-
Exercise 10-8 Utilization of a constrained Resource [LO10-5, L010-6] Barlow Company manufactures three products: A, B, and C. The selling price, variable costs, and contribution margin for one unit...

Study smarter with the SolutionInn App


