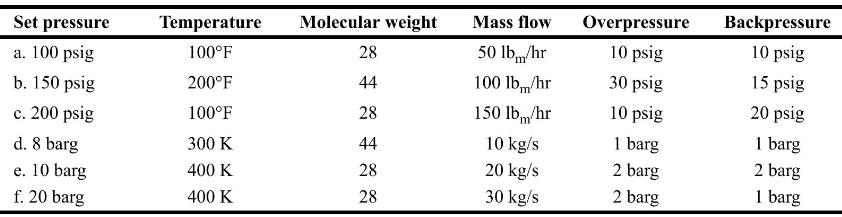
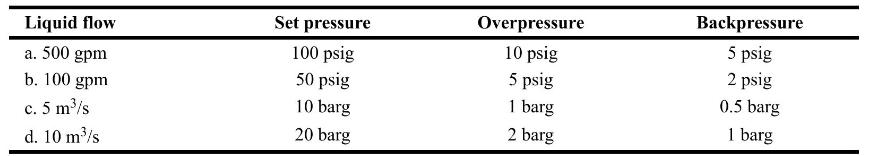
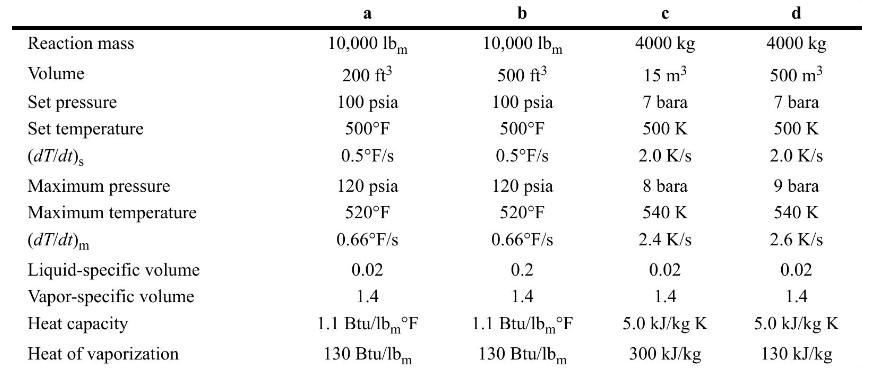
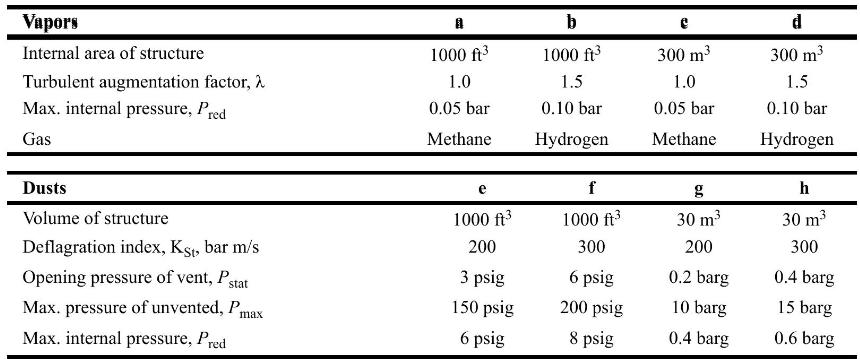
Chemical Process Safety Fundamentals With Applications 4th Edition Daniel A. Crowl, Joseph F. Louvar - Solutions
Explore the comprehensive world of "Chemical Process Safety Fundamentals With Applications 4th Edition" by Daniel A. Crowl and Joseph F. Louvar with our expertly crafted solutions. Access online answers key and solutions in PDF format, featuring solved problems and step-by-step answers. Dive deep into chapter solutions and test banks designed for thorough understanding. Whether you're seeking a solution manual, instructor manual, or free download options, our resourceful textbook answers all your questions. Enhance your learning experience with detailed questions and answers, ensuring a solid grasp on chemical process safety principles.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()



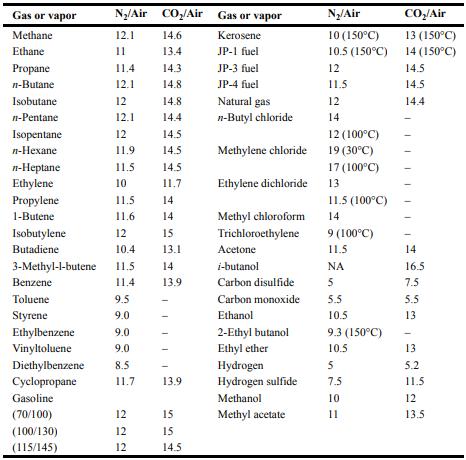
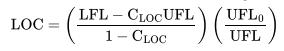
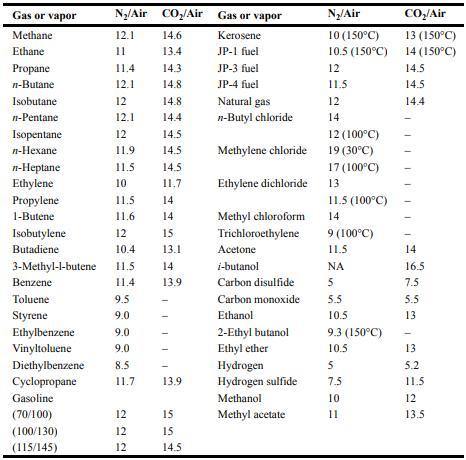
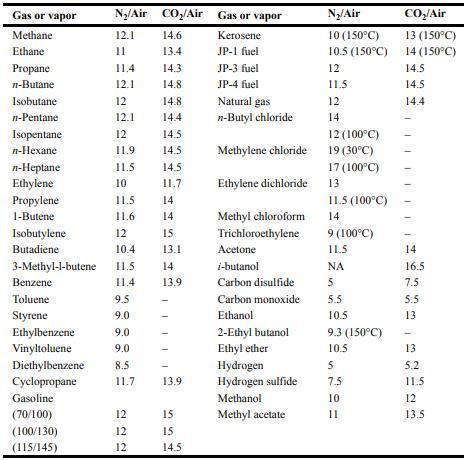
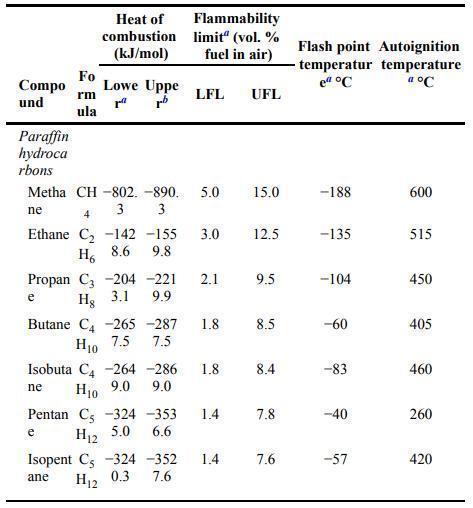
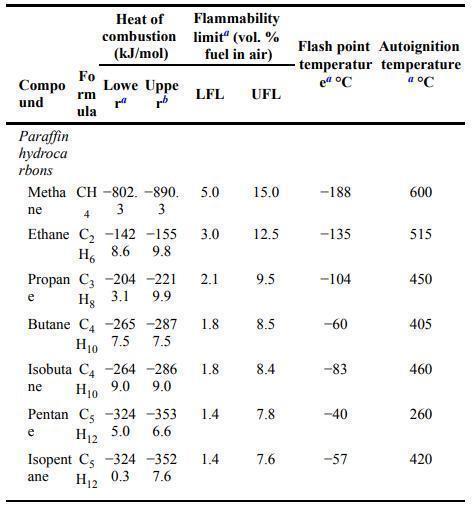
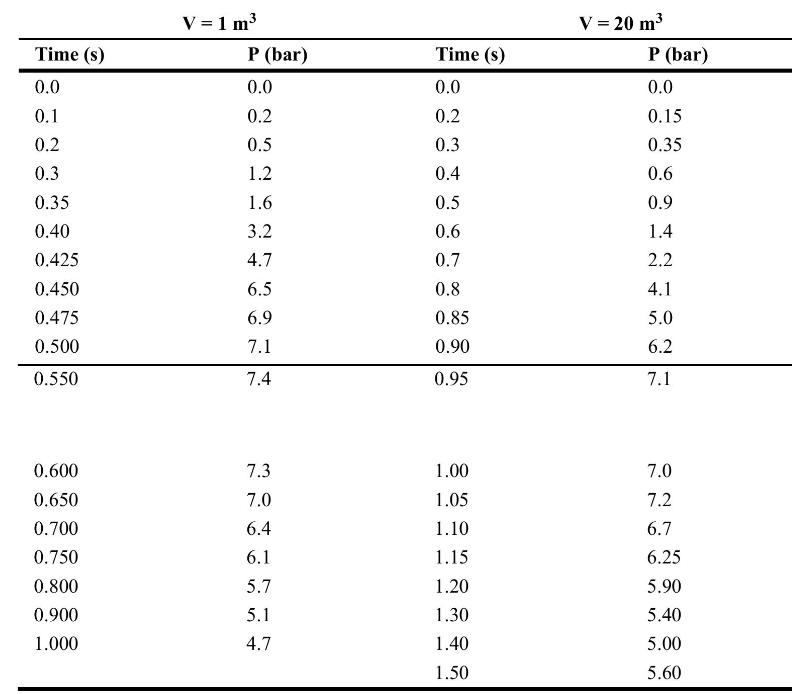
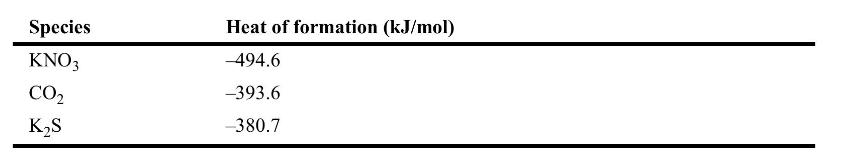
![Im = 1 2nB [-(T -(T+2n)+T [T+4n(1 + B)] (8-21)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/9/9/9/23365af7b81826771705999232078.jpg)
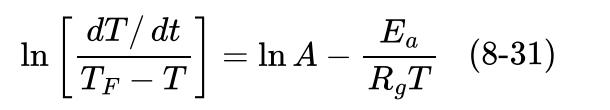
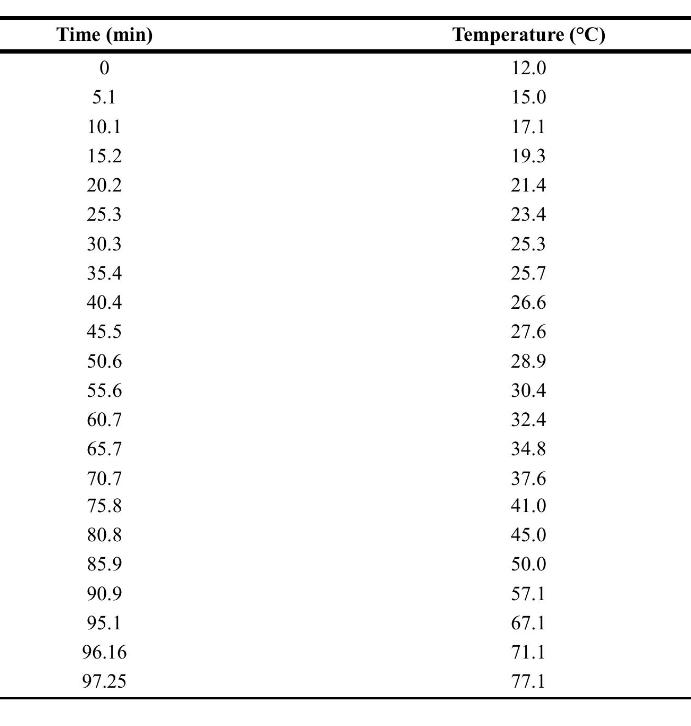
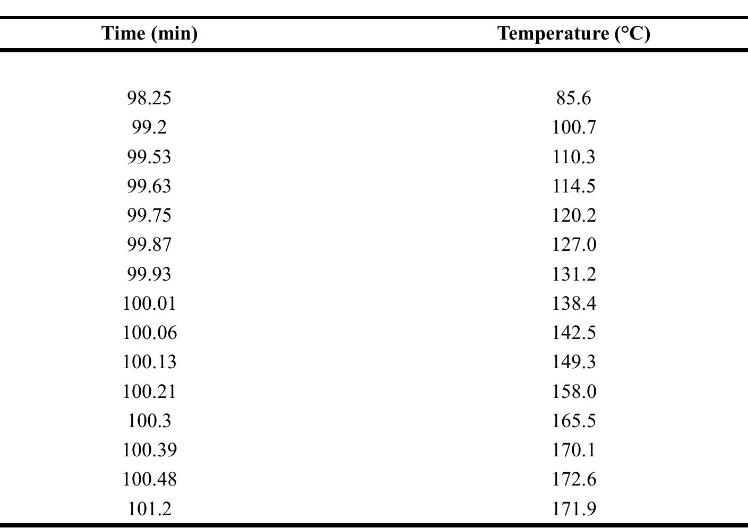
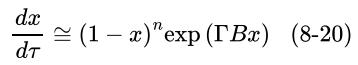
![xm 1 2n B -(T + 2n)+T [T+4n(1 + B)] (8-21)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1706/0/0/1/59965af84bfb3a9f1706001598683.jpg)