Determine the relief diameter for the following two-phase flow conditions. Assume in all cases that (L /
Question:
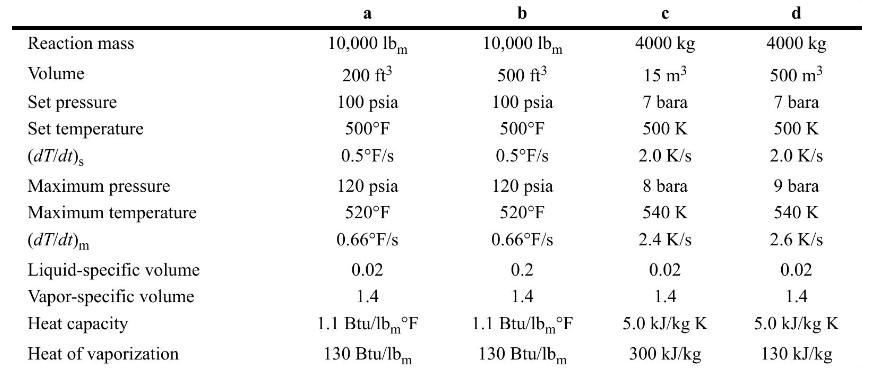
Determine the relief diameter for the following two-phase flow conditions. Assume in all cases that \(L / D=0.0\).
Transcribed Image Text:
Reaction mass Volume Set pressure Set temperature (dT/dt)s Maximum pressure Maximum temperature (dT/dt)m Liquid-specific volume Vapor-specific volume Heat capacity Heat of vaporization a 10,000 lbm 200 ft 100 psia 500F 0.5F/s 120 psia 520F 0.66F/s 0.02 1.4 1.1 Btu/lbF 130 Btu/lbm b 10,000 lbm 500 ft 100 psia 500F 0.5F/s 120 psia 520F 0.66F/S 0.2 1.4 1.1 Btu/lb F 130 Btu/lbm 4000 kg 15 m 7 bara 500 K 2.0 K/s 8 bara 540 K 2.4 K/s 0.02 1.4 5.0 kJ/kg K 300 kJ/kg d 4000 kg 500 m 7 bara 500 K 2.0 K/s 9 bara 540 K 2.6 K/s 0.02 1.4 5.0 kJ/kg K 130 kJ/kg
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To determine the relief diameter for twophase flow conditions you need to use a method that can account for the change in fluid properties and mass fl...View the full answer

Answered By

Talha Talib
I am a member of IEEE society. As i am a student of electrical engineering badge 17 but beside of this i am also a tutor in unique academy. I teach calculus, communication skills, mechanics and economics. I am also a home tutor. My student Muhammad Salman Alvi is a brilliant A-level student and he performs very well in academics when i start to teach him. His weak point was mathematics but now he is performing well in mathematics. I am a scholarship holder in Fsc as i scored 1017 marks in metric out of 1100. Later on i got scholarship in Punjab Group of Colleges. I got 2nd position in robotics competition in 2018 as my project home automation select for the exhibition in Expocentre.
4.60+
23+ Reviews
62+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
(a) LEP Table 12-2: Exothermic Reaction with Heat Exchange Download the Polymath, MATLAB, Python, or Wolfram codes for the algorithm and data given in Table T12-2 for the exothermic gas phase...
-
The table below itemizes several assets. The company uses the double declining balance method to determine depreciation expense, which means that the rate used will be 2 divided by useful life. For...
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
/* FILE: FLIX2YOU_data-load.txt */ /* Script to populate tables for FLIX2YOU .. current schema before revision */ /* Written by Gary Heberling on July 2, 2012 */ /* For IST210 world campus Penn State...
-
For the machine element shown, locate the x coordinate of the center of gravity. ask.
-
Suppose the gold spot price is $300/oz, the 1-year forward price is 310.686, and the continuously compounded risk-free rate is 5%. a. What is the lease rate? b. What is the return on a cash-and-carry...
-
Mobile access to social media. The Marketing Management Journal (Fall 2014) published the results of a designed study to investigate satisfaction with the use of mobile devices to access social...
-
Lansing Electronics Inc. manufactures a variety of printers, scanners, and fax machines in its two divisions: the PSF Division and the Components Division. The Components Division produces electronic...
-
steamer has 6 years of remaining life. If kept, the steamer will have de precierit expens 5 5 0 or he next 5 years ( year 1 through year 5 ) and $ 5 0 0 for the sixth year. Its current book value is...
-
Determine the deflagration vent size for the following structures: Vapors Internal area of structure Turbulent augmentation factor, Max. internal pressure, Pred Gas Dusts Volume of structure...
-
Calculate the diameter for rupture discs in vapor service for the following conditions. Assume that nitrogen is the vent gas. Gas flow a. 100 lb/hr b. 200 lb/hr c. 10 kg/s d. 20 kg/s Temperature 100F...
-
What is the effect of a guard condition?
-
A release has been planned with 5 sprints. The team, for the sake of convenience, has decided to keep the sprint duration open. Depending on how much they commit and achieve, they decide to wrap up...
-
Task 3: Reach-truck management 3 Explain why battery-powered reach truck activities at PAPFS are unsatisfactory. Note: You should support your answer, where applicable, using relevant information...
-
Exercise 6: Black Pearl, Inc., sells a single product. The company's most recent income statement is given below. Sales $50,000 Less variable expenses Contribution margin Less fixed expenses Net...
-
Your maths problem x+3x-3
-
Spencer is a 10-year-old boy who has been living in a family-style therapeutic group home for one year. He was removed from his mother's care due to neglect from her drug use and the resulting legal...
-
A sample of 20 chocolate chip cookies was taken from a box. The cookies were examined for the number of chocolate chips in each cookie. The numbers recorded were 18 15 17 17 16 18 16 15 16 14 16 17...
-
Maria Castigliani is head of the purchasing department of Ambrosiana Merceti, a medium-sized construction company. One morning she walked into the office and said, The main problem in this office is...
-
Consider the titration of 25.0 mL of 0.050 0 M Sn 2+ with 0.100 M Fe 3+ in 1 M HCl to give Fe 2+ and Sn 4+ , using Pt and calomel electrodes. (a) Write a balanced titration reaction. (b) Write two...
-
What is a Jones reductor and what is it used for?
-
Why is iodine almost always used in a solution containing excess I - ?
-
Ray Company provided the following excerpts from its Production Department's flexible budget performance report. Required: Complete the Production Department's Flexible Budget Performance Report....
-
Problem 1 5 - 5 ( Algo ) Lessee; operating lease; advance payment; leasehold improvement [ L 0 1 5 - 4 ] On January 1 , 2 0 2 4 , Winn Heat Transfer leased office space under a three - year operating...
-
Zafra and Stephanie formed an equal profit- sharing O&S Partnership during the current year, with Zafra contributing $100,000 in cash and Stephanie contributing land (basis of $60,000, fair market...

Study smarter with the SolutionInn App


