Estimate the LOC of ethylene using Equations 6-15 and 6-16 in the textbook. Compare to the experimental
Question:
Estimate the LOC of ethylene using Equations 6-15 and 6-16 in the textbook. Compare to the experimental value in Table 6-3.
Table 6-3:
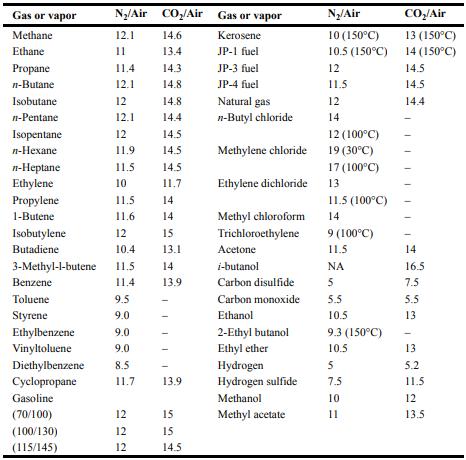
Equation 6-15:
![]()
Equation 6-16:
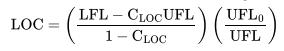
Transcribed Image Text:
Gas or vapor Methane Ethane Propane n-Butane Isobutane n-Pentane Isopentane n-Hexane 12 11.9 11.5 10 11.5 11.6 12 10.4 3-Methyl-l-butene 11.5 11.4 n-Heptane Ethylene Propylene 1-Butene Isobutylene Butadiene Benzene Toluene Styrene Ethylbenzene Vinyltoluene N/Air CO/Air 12.1 14.6 11 13.4 11.4 14.3 12.1 14.8 12 14.8 12.1 14.4 14.5 14.5 Gasoline (70/100) (100/130) (115/145) 9.5 9.0 9.0 9.0 Diethylbenzene 8.5 Cyclopropane 11.7 12 222 12 12 14.5 11.7 14 14 15 13.1 14 13.9 1 13.9 15 15 14.5 Gas or vapor Kerosene JP-1 fuel JP-3 fuel JP-4 fuel Natural gas n-Butyl chloride Methylene chloride Ethylene dichloride Methyl chloroform Trichloroethylene Acetone i-butanol Carbon disulfide Carbon monoxide Ethanol 2-Ethyl butanol Ethyl ether Hydrogen Hydrogen sulfide Methanol Methyl acetate N/Air 10 (150C) 10.5 (150C) 12 11.5 12 14 12 (100C) 19 (30C) 17 (100C) 13 11.5 (100C) 14 9 (100C) 11.5 5 5.5 10.5 9.3 (150C) 10.5 5 7.5 10 11 COy/Air 13 (150C) 14 (150C) 14.5 14.5 14.4 1 14 16.5 7.5 5.5 13 13 5.2 11.5 12 13.5
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
Equations 615 and 616 can be used to estimate the Limiting Oxygen Concentration LOC of ethylene by u...View the full answer

Answered By

Mercy Kangai
I provide creative and detailed administrative, web search, academic writing, data entry, Personal assistant, Content writing, Translation, Academic writing, editing and proofreading services. I excel at working under tight deadlines with strict expectations. I possess the self-discipline and time management skills necessary to have served as an academic writer for the past seven years. I can bring value to your business and help solve your administrative assistant issues. I have extensive experience in marketing and small business management.
4.80+
27+ Reviews
86+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
Estimate the LOC for (a) carbon monoxide and (b) heptane using Equation 6-15. Compare to experimental values in Table 6-3. Table 6-3: Equation 6-15: Gas or vapor Methane Ethane Propane n-Butane...
-
In a study of hypnotic suggestion, 16 male volunteers were randomly allocated to an experimental group and a control group. Each subject participated in a two-phase experimental session. In the first...
-
To calculate variance and standard deviation, we take the deviations from the mean. At times, we need to consider the deviations from a target value rather than the mean. Consider the case of a...
-
The following scatter plot indicates that 200 150 > 100 50 0 0 20 X 40 O a log x transform may be useful Oa y transform may be useful a x transform may be useful Ono transform is needed Oa 1/x...
-
Determine the volume and the surface area of the chain link shown knowing that it is made from a 0.5-in.-diameter bar and that R = 0.75in and L = 3in.
-
Black Ltd., a Canadian private corporation, owns 40% of the voting shares of White Ltd. In the current year, White paid a dividend of $60,000, which triggered a dividend refund of $20,000 to White....
-
According to New Jersey Business (Feb. 1996), Newark International Airport's new terminal handles an average of 3,000 international passengers an hour, but is capable of handling twice that number....
-
On January 1, 2010, Parker, Inc., a U.S.-based firm, acquired 100 percent of Suffolk PLC located in Great Britain for consideration paid of 52,000,000 British pounds (£), which was equal to...
-
(10 points) A) An engineer takes a loan form a lending company equals to $10,000. The lending company takes X % annual interest rate compounded quarterly. The engineer agrees to pay off the loan in...
-
Draw an approximate flammability triangle diagram for methyl alcohol. Use published flammability data from Appendix B and Table 6-3. If a gas containing 20\% methyl alcohol, \(5 \%\) oxygen, and \(75...
-
Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11 in the textbook. Compare to the experimental values in Appendix B. Data From Appendix B:...
-
Bee Company shows the following account amounts: Determine how much cash was generated from sales during 2011. 2011 2012 Sales S8,743,000 7,945,000 Accounts Receivable. 1231 459.000
-
1. Make a comparison between the leadership approaches "Trait Models" and "Behavioral Models". Discuss the main postulates and differences between the models and provide examples of a theory...
-
Applying the Central Limit Theorem: The amount of contaminants that are allowed in food products is determined by the FDA ( Food and Drug Administration ) . Common contaminants in cow milk include...
-
A collection of techniques used by social scientists to compile, summarize, and convey numerical data. Revised Research Question Hypothesis : Null Hypothesis : A null hypothesis, often known as H0,...
-
Adidas is an international sporting apparel/shoes brand. If Adidas was to enter a new foreign market, it would conduct a country market assessment. Identify the 4 components of the assessment....
-
Using the scenario linked in the Supporting Materials section, assume that you are the cost accountant for your company, and the CFO has asked for your analysis on purchasing materials from an online...
-
A local power commission sent a survey to homeowners to determine household power efficiency. Following is a list of major electrical appliances and their total kilowatt-hour (kWh) usage for one...
-
A consumer magazine is evaluating five brands of trash compactors for their effectiveness in reducing the volume of typical household products that are discarded. In the experiment, each block...
-
The amount of power transmitted by sunlight depends on latitude and the surface area of the solar collector. On a clear day at a certain northern latitude, 0.6 kW/m 2 of solar power strikes the...
-
The property of a fluid called viscosity is related to its internal friction and resistance to being deformed. The viscosity of water, for instance, is less than that of molasses and honey, just as...
-
Referring to the description in Problem P3.16, and given that the viscosity of a certain engine oil is 0.25 kg/(m s), determine the value in the units (a) Poise and (b) Slug/(ft s). Problem P3.16...
-
This is a partial adjusted trial batance of Cullumber Compary manualys
-
Which of the following journal entries will record the payment of a $1,500 salaries payable originally incurred for Salaries Expense? Select one: A. Debit Salaries Expense; credit Salaries Payable B....
-
What is the definition of substantially appreciated inventory? A. Inventory with a FMV greater than its basis B. Inventory and unrealized receivables with a FMV greater than their basis C. Inventory...

Study smarter with the SolutionInn App


