Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11
Question:
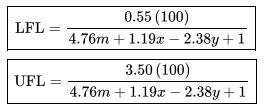
Estimate the UFL and the LFL for ethylene using the stoichiometric concentrations and Equations 6-10 and 6-11 in the textbook. Compare to the experimental values in Appendix B.
Data From Appendix B:
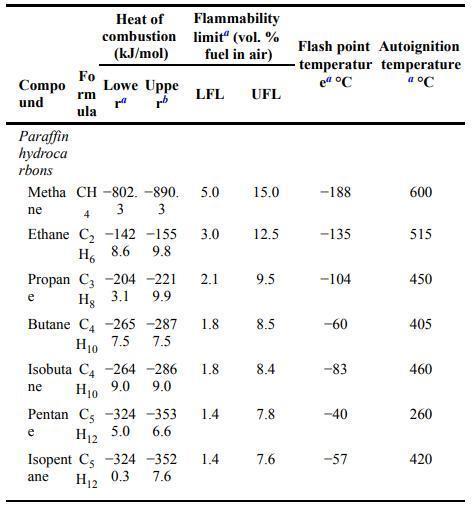
Equation 6-10 and 11:
Transcribed Image Text:
Paraffin hydroca rbons Fo rm ula Compo Lowe Uppe und Heat of combustion (kJ/mol) Flammability limit" (vol. % fuel in air) Metha CH-802. -890. 5.0 ne 3 3 e LFL 4 Ethane C -142 -155 3.0 H 8.6 9.8 Pentan C -324 -353 6.6 H12 5.0 Propan C3 204 -221 2.1 9.9 Hg 3.1 e Butane C4 -265-287 1.8 7.5 H10 7.5 Isobuta C4 -264 -286 1.8 8.4 ne H0 9.0 9.0 1.4 UFL Isopent C -324 -352 1.4 ane H12 0.3 7.6 15.0 12.5 9.5 8.5 7.8 7.6 Flash point Autoignition temperatur temperature el C a C -188 -135 -104 -60 -83 -40 -57 600 515 450 405 460 260 420
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the Lower Flammability Limit LFL and Upper Flammability Limit UFL for ethylene using the given equations we need to know the stoichiometr...View the full answer

Answered By

Joseph Ogoma
I have been working as a tutor for the last five years. I always help students to learn and understand concepts that appears challenging to them. I am always available 24/7 and I am a flexible person with the ability to handle a wide range of subjects.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar
Question Posted:
Students also viewed these Engineering questions
-
a. Example 13-1: Batch Reactor with an Exothermic Reaction Wolfram 1. Adiabatic Case: Use Wolfram to see whether you can find a trajectory that is ready to ignite and whose trajectory looks like a...
-
Estimate the upper and lower flammable limits for carbon monoxide and heptane using the stoichiometric method via Equations 6-10 and 6-11 in the text. Compare to experimental values provided in...
-
Molecular simulation can be used to explore the accuracy and significance of individual contributions to an equation of state. Use the DMD module at Etomica.org to explore Xes energy departure. (a)...
-
A certain apple bruises if a net force greater than 9 . 5 N is exerted on it . Would a 0 . 1 3 k g apple be likely to bruise if it falls 1 . 8 m and stops after sinking 0 . 0 5 m into the grass?...
-
Determine the volume of the solid generated by rotating the semi elliptical area shown about (a) The axis AA², (b) The axis BB², (c) The y axis.
-
A trust is considered to be an individual for tax purposes and therefore its taxation year is the calendar year. Is this statement true? Explain.
-
The Chronicle of Higher Education Almanac (Aug. 27,1999) reports that the percentage of undergraduates in the United States receiving federal financial aid is 45% at public four-year institutions and...
-
Performance Auto Company operates a New Car Division (that sells high performance sports cars) and a Performance Parts Division (that sells performance improve ment parts for family cars). Some...
-
1 ) Start your search in the ABI / Inform Collection ( Database ) . 2 ) Check the box for "Peer Reviewed" to limit the results to peer reviewed articles only. 3 ) Limit the Publication Date to Jan 2...
-
Estimate the LOC of ethylene using Equations 6-15 and 6-16 in the textbook. Compare to the experimental value in Table 6-3. Table 6-3: Equation 6-15: Equation 6-16: Gas or vapor Methane Ethane...
-
A gas cylinder contains a gas mixture composed of \(50 \%\) methane and \(50 \%\) ethylene by volume. Estimate the LFL and the UFL for this gas mixture. Compare to the experimental values of \(3.6...
-
What are the major issues with regard to trade imbalances for low-and middle-income countries?
-
Your introduction needs to include the following. o Include a clear definition of unemployment and inflation and how and why they occur and rise in the economy. o Briefly provide your understanding...
-
Questions: 1. What strategies can be employed to foster a sense of inclusion and belonging within teams, and what are the potential benefits of doing so? 2. How can a team be successful? 3. What is...
-
Critical reflection involves closely examining events and experiences from different perspectives to inform future practice. In a few paragraphs, explain - Why educators should regularly reflect on...
-
What resources does the school or school district provide to teachers to promote diversity, equity, and inclusion? What are some of the strengths and shortcomings of the school's policies on...
-
Select FOUR companies listed on the UK Stock Exchange. Chose two companies from one industry sector and two other companies from another industry sector. By using the most recent three years'...
-
Refer to the data given in Exercise 9, and construct a pie chart. Compare the pie chart to the Pareto chart, and determine which graph is more effective in showing the relative importance of job...
-
Chao, Louis, and Mari, unrelated individuals, own all of the shares of Cerise Corporation. All three shareholders have been active in the management of Cerise since its inception. In the current...
-
Referring to the description in Problem P3.16, if the viscosity of water is 0.01 poise, determine the value in terms of the units (a) Slug/(ft s) (b) kg/(m s). Problem P3.16 The property of a fluid...
-
The fuel efficiency of an aircrafts jet engines is described by the thrust-specific fuel consumption (TSFC). The TSFC measures the rate of fuel consumption (mass of fuel burned per unit time)...
-
An automobile engine is advertised as producing a peak power of 118 hp (at an engine speed of 4000 rpm) and a peak torque of 186 ft lb (at 2500 rpm). Express those performance ratings in the SI...
-
Case Products manufactures two models of DVD storage cases: regular and deluxe. Presented is standard cost information for each model: Cost Components Regular Deluxe Direct materials Lumber 2 board...
-
A corporate bond that you own at the beginning of the year is worth $930. During the year, it pays $56 in interest payments and ends the year valued at $920. What was your dollar return and percent...
-
Anissa makes custom bird houses in her garage and she buys all her supplies from a local lumber yard. Last year she purchased $4500 worth of supplies and produced 2500 bird houses. She sold all 2500...

Study smarter with the SolutionInn App


