A chemical reaction has been characterized via a calorimeter and the following results were obtained: Reaction order,
Question:
A chemical reaction has been characterized via a calorimeter and the following results were obtained:
Reaction order, \(n: 1.00\)
Detected onset temperature, \(T o: 350 \mathrm{~K}\)
Final temperature, \(T F: 612 \mathrm{~K}\)
Activation energy, \(E a: 29.1 \mathrm{~kJ} / \mathrm{mol}\)
Pre-exponential, \(A: 2 \times 10^{-2} \mathrm{~s}^{-1}\)
a. What are the values of the dimensionless parameters \(B\) and \(\Gamma\)
b. What is the maximum temperature rate \((\mathrm{K} / \mathrm{s})\) and at what temperature does this occur \((\mathrm{K})\) ?
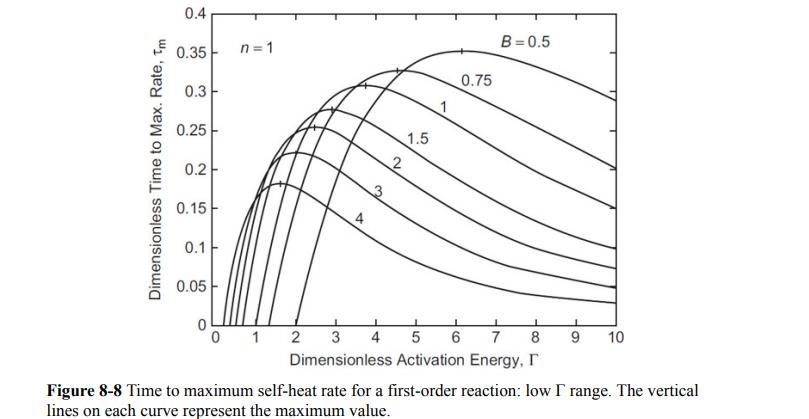
c. How many seconds after the detected onset temperature does the maximum temperature rate occur? (Hint: Use Figure 8-8.)
Step by Step Answer:

Chemical Process Safety Fundamentals With Applications
ISBN: 9780134857770
4th Edition
Authors: Daniel A. Crowl, Joseph F. Louvar





