Answered step by step
Verified Expert Solution
Question
1 Approved Answer
3. (5 points) The uncertainty principle limits our ability to determine simultaneously the position and momentum of a particle. (a) Why were classical physicists
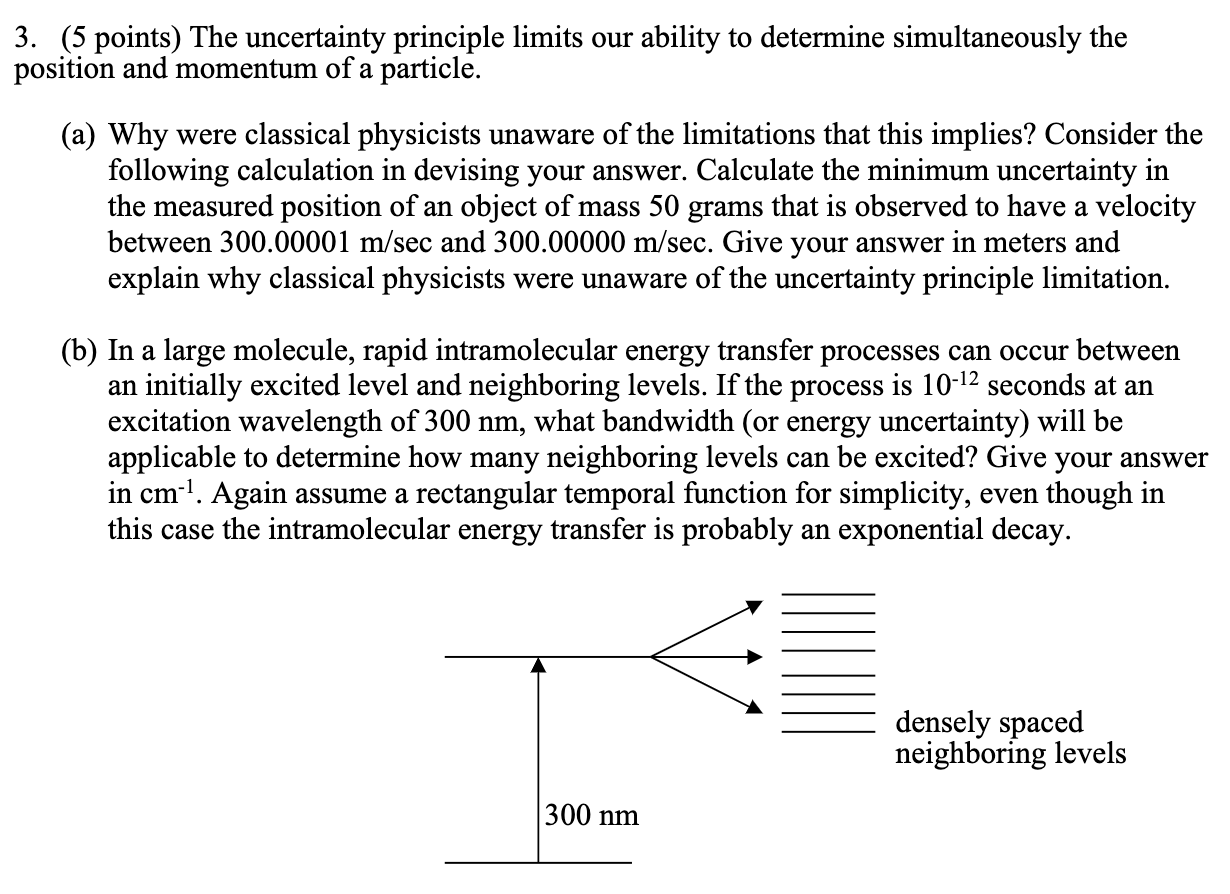
3. (5 points) The uncertainty principle limits our ability to determine simultaneously the position and momentum of a particle. (a) Why were classical physicists unaware of the limitations that this implies? Consider the following calculation in devising your answer. Calculate the minimum uncertainty in the measured position of an object of mass 50 grams that is observed to have a velocity between 300.00001 m/sec and 300.00000 m/sec. Give your answer in meters and explain why classical physicists were unaware of the uncertainty principle limitation. (b) In a large molecule, rapid intramolecular energy transfer processes can occur between an initially excited level and neighboring levels. If the process is 10-12 seconds at an excitation wavelength of 300 nm, what bandwidth (or energy uncertainty) will be applicable to determine how many neighboring levels can be excited? Give your answer in cm. Again assume a rectangular temporal function for simplicity, even though in this case the intramolecular energy transfer is probably an exponential decay. 300 nm densely spaced neighboring levels
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solution Given Mass m50grams005kg Velocity uncertainty v30000001ms30000000ms000001ms Time duration t ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


