A step tracer input was used on a real reactor with the following results: a. What is
Question:
A step tracer input was used on a real reactor with the following results: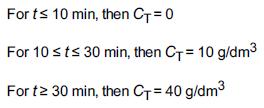
a. What is the mean residence time tm?
b. What is the variance σ2?
Transcribed Image Text:
For t≤ 10 min, then C₁ = 0 For 10 st≤ 30 min, then C+= 10 g/dm³ For t≥ 30 min, then C+= 40 g/dm³
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a The cumulative distributio...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Engineering questions
-
Doing the computations by hand, find the determinant of the matrix below. 6 2
-
Answer Exercise 1 for the case where α is replaced by 4i 3 2
-
Use the RTD data in Examples 16-1 and 16-2 to predict X PFR , X CSTR , X LFR , X T-I-S , X seg and X mm for the following elementary gas-phase reactions a. A B k = 0.1 min 1 b. A 2B k = 0.1 min 1...
-
Solve the right triangles with the given parts or state that there is not enough information to solve. Round off results according to Table 4.1. Refer to Fig. 4.37. B = 32.1, c = 238 Data from Table...
-
In July 1988, Chester Crow executed a promissory note payable to the order of THE FIRST NATIONAL BANK OF SHREVEPORT or BEARER in the amount of $21,578.42 at an interest rate of 3 percent per year...
-
How might they integrate other channels into their overall selling model?
-
approximately 4000 in four years time. Assuming that Valerie adopts the straight-line method of depreciation in her accounts what is her depreciation charge for the accounting year ending 31 December...
-
The Star Exploration Agency, a unit of the Space Department, was established by Congress to begin operations at the beginning of fiscal year 2014. Following are the agencys transactions during...
-
At an interest rate of 4%, what comes closest to the amount of time it takes for a deposit of $7,500 to double, that is, to reach $15,000? A. 4.00 years B. 10.24 years C. 2.00 years D. 17.67 years E....
-
Files and More, Inc. (F&M), a manufacturer of office equipment, uses MRP to schedule its production. Because of the current recession and the need to cut costs, F&M has targeted inventory as a prime...
-
The following E(t) curves were obtained from a tracer test on two tubular reactors in which dispersion is believed to occur. (a) RTD Reactor A; (b) RTD Reactor B The graphs for RTD reactors A and B...
-
Consider the E(t) curve below. A graph is shown, with t (in minutes) on horizontal axis and E of t (minutes inverse) on vertical axis. A hemi (half) circular curve starts at the origin and ends at 2...
-
Forten Companys current-year income statement, comparative balance sheets, and additional information follow. For the year, (1) All sales are credit sales, (2) All credits to Accounts Receivable...
-
5 Informatics solutions in the "complex and catastrophic" end of the population-risk spectrum must support which type of services/functions? 1 point Intensive case management Wellness program
-
What are the characteristics of products that Otis Trains produces? What are order qualifiers and winners? Explain at least three advantages and three drawbacks of offshoring to JLPTC. What risks are...
-
Find the angle and length of the resulting vector for the given d and e vectors by the analytical method. After that, find the parameters of the resulting vector for the three vectors. In the answer,...
-
The problem I have identified is that healthcare leaders could benefit from addressing the issue of stress and burnout, which impact revenue (Scott, 2022). I have found a peer-reviewed article...
-
Facebook, Inc is the company Complete a 3-5 year forecast for your target company assuming a 10% average growth rate for the duration of the forecast period Assuming a long-term growth rate of 5%...
-
Rachel, Pete, and Brian perform Part 2 of the investigation in this lesson. Rachel walks while Pete and Brian hold the motion sensors. They create the unusual graph at right. The horizontal axis has...
-
A glass manufacturer produces hand mirrors. Each mirror is supposed to meet company standards for such things as glass thickness, ability to reflect, size of handle, quality of glass, color of...
-
Why do we say that the equilibrium constant for the reaction H 2 O H + + OH - (or any other reaction) is dimensionless?
-
Write the expression for the equilibrium constant for each of the following reactions. Write the pressure of a gaseous molecule, X, as P X . a. b. 3Ag*(aq) + PO (aq) = Ag3PO4(s)
-
(a) A favorable entropy change occurs when S is positive. Does the order of the system increase or decrease when S is positive? (b) A favorable enthalpy change occurs when H is negative. Does the...
-
9. The following selected data are from a recent statement of cash flows: Net cash flow from operating activities $35,000 Net cash flow used for investing activities (20,000) Net cash flow from...
-
You can handle the 20+ tax system expense categories and multiple classes of operating expenses (COR, COG, SGA) by using account numbers with at least ________ digits .
-
is the obligation that is created when an employee accepts a manager's delegated authority

Study smarter with the SolutionInn App


