Also Hall of Fame Problem. The irreversible liquid-phase reactions Reaction(1)A + B 2Cr1C = k1CCACBReaction(2)2B+CDr2D=k2DCBCC are
Question:
Also Hall of Fame Problem. The irreversible liquid-phase reactions Reaction(1)A + B → 2Cr1C = k1CCACBReaction(2)2B+C→Dr2D=k2DCBCC
are carried out in a PFR with heat exchange. The temperature profiles shown in Figure P12-21B were obtained for the reactor and the coolant stream: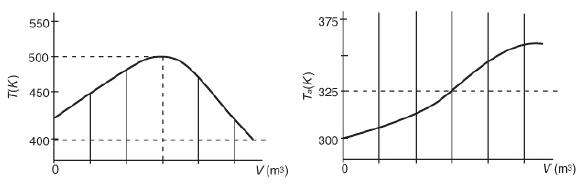
Reactant temperature T and coolant temperature Ta profiles. Two graphs, one plots Reactant temperature (T) against the volume (V) and another plots the coolant temperature (T Subscript a) against the volume (V) are shown. The horizontal axis of the first graph represents V in meter cubed and the vertical axis of the first graph ranges from 400 to 550 in increments of 50. There is a curve
drawn on the graph from the point 430 on the y-axis from where it goes up to 500 and reaches a midpoint. From there it drops down to the value of 400. The points where the values of y-axis meets the x-axis is marked using dotted lines. The lowest point of the curve is said to be 400 whereas the highest point is said to be 500. The horizontal axis of the second graph represents V in meter cubed and the vertical axis of the second graph ranges from 300 to 375 in increments of 25. There is a curve drawn on the graph from the point 300 on the y-axis from where it goes up to 325 and reaches the midpoint. From there it further goes up to the value of 350. The points where the values of y-axis meets the x-axis is marked using straight lines. The lowest point of the curve is observed to be 400 whereas the highest point is observed to be 500. And the mid value 325 is marked using a dotted line. Note that the values are approximate. The concentrations of A, B, C, and D were measured at the point down the reactor where the liquid temperature, T, reached a maximum, and they were found to be CA
= 0.1, CB = 0.2, CC = 0.5, and CD = 1.5, all in mol/dm3. The product of the overall heat transfer coefficient and the heat-exchanger area per unit volume, Ua, is 10 cal/s ·
dm3 · K. The entering molar flow rate of A is 10 mol/s.
Additional information: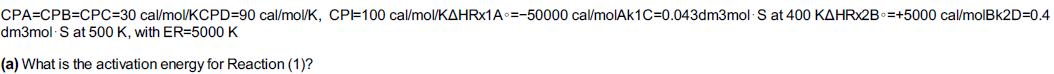
Step by Step Answer:






