The apparatus in the figure can follow the course of an EDTA titration and was used to
Question:
The apparatus in the figure can follow the course of an EDTA titration and was used to generate the curves in Figure 11-10. The heart of the cell is a pool of liquid Hg in contact with the solution and with a Pt wire. A small amount of HgY2- added to the analyte equilibrates with a very tiny amount of Hg2+:
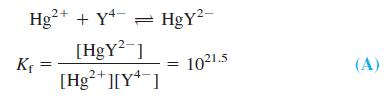
The redox equilibrium 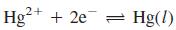 is established rapidly at the surface of the Hg electrode, so the Nernst equation for the cell can be written in the form
is established rapidly at the surface of the Hg electrode, so the Nernst equation for the cell can be written in the form
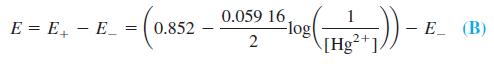
where E- is the constant potential of the reference electrode. From Equation A, [Hg2+] = [HgY2-] / Kf[Y4-], and this can be substituted into Equation B to give
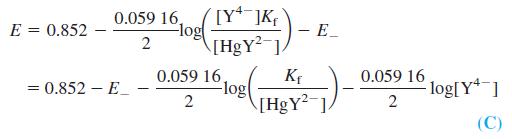
where Kf is the formation constant for HgY2-. This apparatus thus responds to the changing EDTA concentration during an EDTA titration.
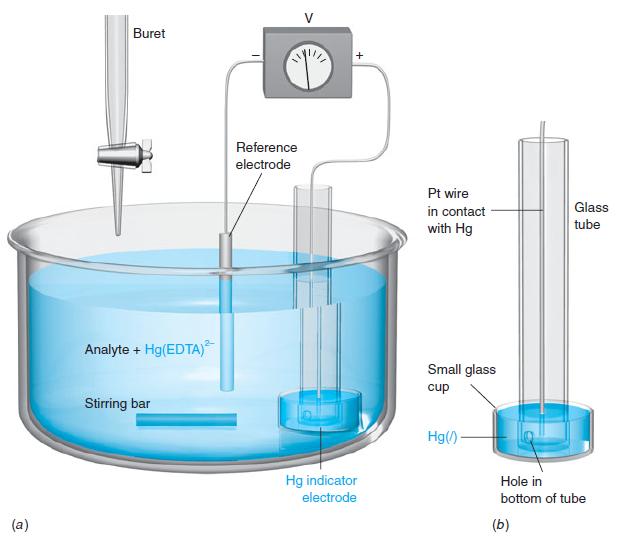
Suppose that you titrate 50.0 mL of 0.010 0 M MgSO4 with 0.020 0 M EDTA at pH 10.0, using the apparatus in the figure with an S.C.E. reference electrode. Analyte contains 1.0 × 10-4 M Hg(EDTA)2- added at the beginning of the titration. Calculate the cell voltage at the following volumes of added EDTA, and draw a graph of millivolts versus milliliters: 0, 10.0, 20.0, 24.9, 25.0, and 26.0 mL.
Step by Step Answer:






