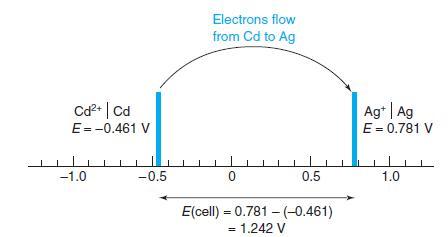
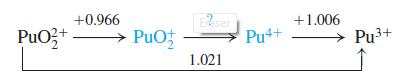
Quantitative Chemical Analysis 8th edition Daniel C. Harris - Solutions
Unlock the potential of your studies with our comprehensive resource for "Quantitative Chemical Analysis 8th Edition" by Daniel C. Harris. Access an extensive collection of solved problems and step-by-step answers with our online solutions manual. Enhance your learning experience with the answers key and solutions PDF, providing a deeper understanding of complex questions and answers. Our test bank and chapter solutions offer invaluable support for exam preparation. Benefit from the instructor manual and textbook insights, all available for free download. Elevate your knowledge with our expertly crafted solutions and answers.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()


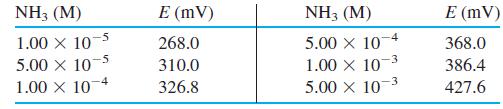
![Po Xo (М) [X] (M) Experiment (М) A 0.010 0 0.213 4.42 x 10 9.10 X 10 1.60 X 10 2.47 X 10 3.57 X 10 1 0.010 0 0.001 00 0.303 2 0.010 0 0.002 00 0.394 -5 3 0.010 0 0.003 00 0.484 -5 4 0.010 0 0.004](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1592/1/2/8/5435ee5f41fec0c51592128542635.jpg)

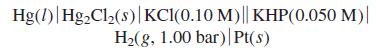
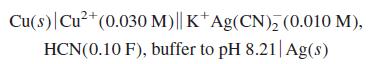
![[M]ree + Kf[M]irce 1 + K#[M]free СЕDTA VEDTA См CMVM [M]ree + K#[Mree K* [M]free + CEDTA](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/7/4/4005ee21360ee36d1591874400228.jpg)
![CH,CO,H H,Ñ CH,CO,H H,X* [X?-1 ax? [H;X*]+ [H2X] + [HX¯] + [X ] Cu²+ + 2x?- = CuX K = B2 = 3.5 × 1016](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/7/4/0055ee211d5cd1f31591874005099.jpg)