Question: (a) Using Reactions 2 and 3, calculate E and write the Nernst equation for the cell. (b) Use the value of K sp for A
(a) Using Reactions 2 and 3, calculate E° and write the Nernst equation for the cell.
(b) Use the value of Ksp for AgI to compute [Ag+] and find the cell voltage. By the reasoning in Figure 13-8, in which direction do electrons flow?
(c) Suppose, instead, that you wish to describe the cell with Reactions 1 and 3. We know that the cell voltage (E, not E°) must be the same, no matter which description we use. Write the Nernst equation for Reactions 1 and 3 and use it to find E° in Reaction 1.
Compare your answer with the value in Appendix H.
Figure 13-8
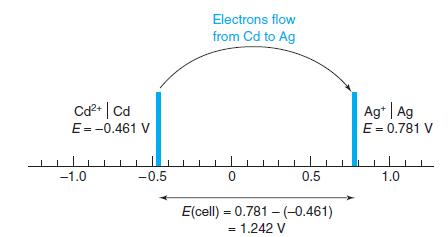
Electrons flow from Cd to Ag Cd?* | Cd Ag* | Ag E = 0.781 V E=-0.461 V -1.0 -0.5 0.5 1.0 E(cell) = 0.781 (-0.461) = 1.242 V
Step by Step Solution
3.46 Rating (166 Votes )
There are 3 Steps involved in it
a The reactions occurring in the cell are Ags Iaq AgIs 1 AgIs Agaq Iaq 2 The standard reduction pote... View full answer

Get step-by-step solutions from verified subject matter experts


