Question: Complex formation by 3-aminopyridine and picric acid in chloroform solution gives a yellow product with an absorbance maximum at 400 nm. Neither starting material absorbs
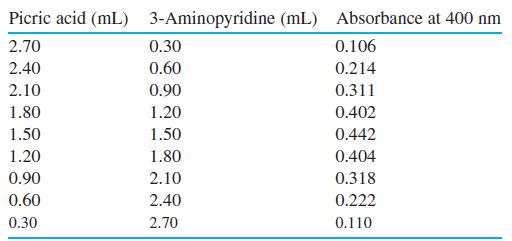
Complex formation by 3-aminopyridine and picric acid in chloroform solution gives a yellow product with an absorbance maximum at 400 nm. Neither starting material absorbs significantly at this wavelength. Stock solutions containing 1.00 × 10-4 M of each compound were mixed as indicated, and the absorbances of the mixtures were recorded. Prepare a graph of absorbance versus mole fraction of 3-aminopyridine and find the stoichiometry of the complex.
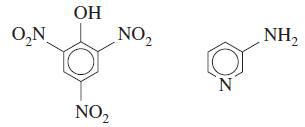
OH O,N NO, NH, NO,
Step by Step Solution
3.34 Rating (145 Votes )
There are 3 Steps involved in it
The mole fraction of 3aminopyridine can be calculated as follows Mole fraction of 3a... View full answer

Get step-by-step solutions from verified subject matter experts


