Question: Fractional composition in a diprotic system. Create a spreadsheet with Equations 9-19 through 9-21 to compute the three curves in Figure 9-4. Plot the three
Fractional composition in a diprotic system. Create a spreadsheet with Equations 9-19 through 9-21 to compute the three curves in Figure 9-4. Plot the three curves in a beautifully labeled figure.
Figure 9-4
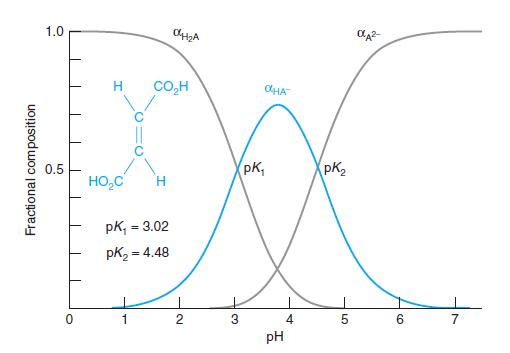
Equations 9-19 to 9-21
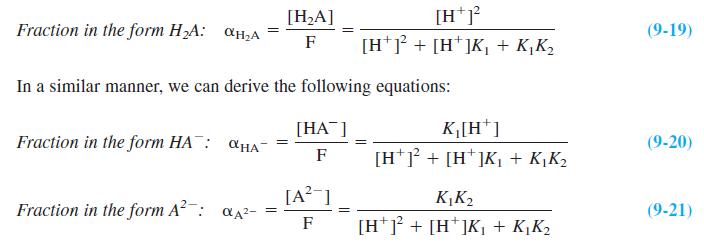
1.0 H CO,H OHA pK, PK, 0.5 HO,C pk, = 3.02 %3! pK2 = 4.48 2 3 4 5. 6. 7 pH Fractional composition
Step by Step Solution
3.34 Rating (157 Votes )
There are 3 Steps involved in it
pH alphaH2A alphaHA alphaA2 0 0999046 0000954 316E08 0070707 0998877 0001123 437E08 0141414 0998679 0001321 606E08 0212121 0998446 0001554 839E08 0282828 0998172 0001828 116E07 0353535 0997849 0002151 ... View full answer

Get step-by-step solutions from verified subject matter experts


