Question: If electrode C in Figure 14-18 were placed in a solution of pH 11.0, what would the pH reading be? Figure 14-18 -1.0 C D
If electrode C in Figure 14-18 were placed in a solution of pH 11.0, what would the pH reading be?
Figure 14-18
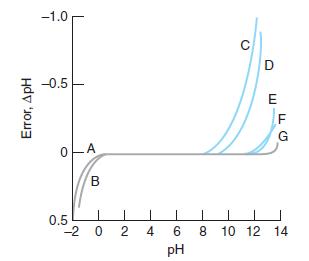
-1.0 C D -0.5 F G B 0.5 -2 0 2 4 6 8 10 12 14 pH Error, ApH
Step by Step Solution
3.31 Rating (166 Votes )
There are 3 Steps involved in it
In Figure 1418 electrode C is the pH electrode which measures the pH of the solution The pH electrod... View full answer

Get step-by-step solutions from verified subject matter experts


