Question: An ammonia gas-sensing electrode gave the following calibration points when all solutions contained 1 M NaOH. A dry food sample weighing 312.4 mg was digested
An ammonia gas-sensing electrode gave the following calibration points when all solutions contained 1 M NaOH.
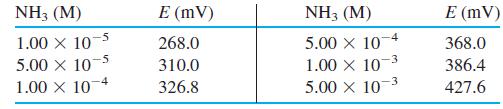
A dry food sample weighing 312.4 mg was digested by the Kjeldahl procedure (Section 10-8) to convert all nitrogen into NH4+. The digestion solution was diluted to 1.00 L, and 20.0 mL were transferred to a 100-mL volumetric flask. The 20.0-mL aliquot was treated with 10.0 mL of 10.0 M NaOH plus enough NaI to complex the Hg catalyst from the digestion and diluted to 100.0 mL. When measured with the ammonia electrode, this solution gave a reading of 339.3 mV. Calculate the wt% nitrogen in the food sample.
NH3 (M) E (mV) NH3 (M) E (mV) -5 -4 1.00 X 10 5.00 x 10 1.00 X 104 5.00 x 10 1.00 x 10-3 5.00 X 103 268.0 368.0 310.0 386.4 326.8 427.6
Step by Step Solution
3.38 Rating (167 Votes )
There are 3 Steps involved in it
To calculate the weight percent nitrogen wt N in the food sample we need to determine the concentrat... View full answer

Get step-by-step solutions from verified subject matter experts


